Fig. 1.1
Evolution and development of topical corticosteroids
1.8 Development of the Assay Methods: Scientific Evaluation Was Inevitable
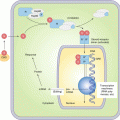
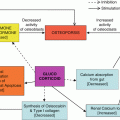
The development of new topical agents inevitably brought the question of comparison of potency and efficacy. Several assay methods were used to compare the potency of the corticosteroid preparations. There was no single specific method to assay the unique therapeutic potentials of corticosteroids. Therefore, a battery of assays were utilized to screen the compounds for further testing. Fibroblast assay, thymus involution assay, anti-granuloma assay and rat ear assay were some of them. Human vasoconstrictor assay, described by McKenzie and Stoughton in 1962, eventually became one of the most useful assay methods for evaluation of potency of topical corticosteroid [27, 28]. The ability of producing vasoconstriction was found to be related to anti-inflammatory effect in the therapeutic arena [29]. Another significant method of assay which was used to evaluate topical corticosteroids was psoriasis small plaque bioassay [30]. However, the contribution of the vasoconstrictor assay in the evaluation of corticosteroid remained a benchmark for a long time [4].
Conclusion
Topical corticosteroid is a milestone that revolutionized the management of several types of dermatoses. The journey which started in 1930 with the adrenal extracts, finally found its track in the road of topical dermatoses in 1952 with hydrocortisone. The journey always remained challenging. This journey was mostly by manipulation of the steroid molecule to produce compounds with greater lipophilicity, fewer mineralocorticoid properties and high potency. However, addition of new members in the regiment was encouraging, and several new topical agents were designed over the years. In the 1960s, the world witnessed the emergence of the high-potency fluorinated agents like triamcinolone, flurandrenolone, flumethasone or betamethasone. Betamethasone was used topically in for the first time in 1964. It was a discovery which remained as a milestone in the dermatology practice. Unfortunately, adverse drug reactions occur by the same mechanism which resulted in the action of the topical corticosteroids. On the other hand, systemic absorption of the topical agents is another challenge which needs to be addressed. Unless these limitations are overcome, the painstaking journey of development of the topical corticosteroids is yet to be over. Therefore, the world will wait to see the better tomorrows with newer topical agents which will virtually eliminate the risk of systemic absorption and adverse effects one fine morning!
References
1.
WHO model prescribing information: drugs used in skin diseases: annex: classification of topical corticosteroids [Internet]. [cited 13 Jan 2017]. Available from: http://apps.who.int/medicinedocs/en/d/Jh2918e/32.html#Jh2918e.32.1.
2.
Must Know Events that Occurred in the 1920s [Internet]. About.com Education. [cited 13 Jan 2017]. Available from: http://history1900s.about.com/od/timelines/tp/1920timeline.htm.
3.
4.
5.
Benedek TG. History of the development of corticosteroid therapy. Clin Exp Rheumatol. 2011;29(5 Suppl 68):S-5–12.
6.
Murray JR. The history of corticosteroids. Acta Derm Venereol Suppl (Stockh). 1989;151:4–6; discussion 47–52.
7.
Kendall EC. Hormones of the adrenal cortex. Bull N Y Acad Med. 1953;29(2):91–100.PubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree