Fig. 5.1
Intraoperative view of anastomosis
When faced with significant rectal bleeding immediately after surgery, the surgeon is left with two options: endoscopic therapy or reoperation. Some surgeons may be concerned about the safety of colonoscopy immediately after surgery and the potential risk of anastomotic dehiscence. There has been no report of such a problem to date [2, 4]. There are authors who routinely or selectively perform intraoperative endoscopy to assess the anastomotic line, and this has not been associated with increased morbidity [5, 6]. For those who have reported the results of endoscopic therapy for anastomotic bleeding, none of the patients had any anastomotic leak after the procedure [1, 2, 4, 7].
Endoscopy allows therapeutic options such as adrenaline injections, diathermy, and endoclip application [2, 4, 7]. The rate of success of endoscopic therapy can be more than 80 %, [1, 2]; reoperation is reserved for the arrest of hemorrhage in those whose bleeding is not controlled by colonoscopic means. Direct visualization can be extremely helpful in the occasional case where the anastomosis is found not to be the source of the bleeding. Attention then can be focused on finding another cause of the bleeding, which provides a much better option than reoperating and then trying to localize an unknown bleeding source during surgery.
Anastomotic Leak
Clinically significant colorectal or coloanal anastomotic leak rates vary from 0.1 to 9 %. Clinical or radiological leaks occur in about 0.4–19.2 % of cases [8–13]. Treatment options include conservative management, percutaneous drainage of sepsis, and fecal diversion with or without take down of the anastomosis. Those in whom the anastomosis is operatively taken down frequently do not undergo restoration of intestinal continuity [12]. When faced with sepsis in a patient with anastomotic leakage, few surgeons would consider endoscopy for fear of further disrupting the anastomosis and disseminating established sepsis.
Numerous methods have been used to attempt to treat anastomotic leak endoscopically. One novel method involves the use of endoscopic, transanal, vacuum-assisted rectal drainage (the endosponge) for extraperitoneal rectal anastomosis. This has been successful in more than 88 % of patients [14–17], without the need for operative reintervention for leaks from low rectal resections presenting with coincident presacral collections. This method has a relatively slow rate of anastomotic healing but seems to be effective, requiring multiple changes (with or without endoscopic assistance) and with acceptable function after the leak. It also has been utilized after definitive radiation therapy when there is an associated leak [18]. The issue of the endosponge is covered in Chap. 49. The use of transanal endoscopic microsurgery or even endoclips to repair the anastomosis also has been described [19]. A future advance might be the use of covered colonic stents to bridge the anastomotic defect and permit healing [20].
Endoscopic evaluation may be performed either before or during reoperation. It allows for assessment of the viability of the mucosa and definition of the location and the extent of the dehiscence. This might not otherwise be readily visualized during reoperation, especially if the anastomosis is deep in the pelvis. The external aspect also may be obscured by inflamed or infected tissues. This additional knowledge would help determine whether the anastomosis needs to be taken down or just reinforced with additional sutures. Endoscopy also helps the surgeon to assess the degree of fecal loading in the bowel proximal to any defunctioning stoma and helps in deciding if further intraoperative bowel cleansing is needed.
Endoscopy for Late Complications After Colorectal Surgery
Some of the more common problems that may require reoperation include recurrent tumor, pouch failure/pouchitis, anastomotic strictures, and colonic fistulas.
Recurrence of Cancer
The use of colonoscopy solely for the detection of recurrences is limited because the majority of cancer recurrences are extraluminal. Nonetheless, colonoscopy is still important for the detection of metachronous lesions, even if there is no suspicion of cancer recurrence. Positron emission tomography/CT is the best way to detect widespread recurrences; however, locoregional tumor recurrence may occur after colorectal resection of a tumor. Commonly, recurrence starts extraluminally from metastatic nodules, regional lymph nodes, or the pelvic side walls. The recurrent tumor only eventually invades the intestinal lumen. The presence of an intact anastomotic line on endoscopy may be an indication that the recurrence is but a locoregional manifestation of a disseminated recurrence (Fig. 5.2). In this respect, previous anastomotic leakage is one of the risk factors for anastomotic recurrence [11, 21, 22]. Endoscopy and biopsies are necessary to confirm whether the lesion has indeed invaded the lumen and may be used concurrently with other imaging suggestive of locoregional recurrence.
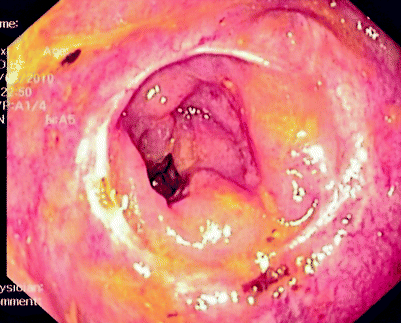

Fig. 5.2
Extraluminal recurrence causing luminal narrowing above previous coloanal anastomosis
Occasionally, local recurrence may start at the anastomotic line. This may be due to luminal implantation of viable shed or trapped cancer cells during the initial colorectal manipulation or anastomosis and is a not uncommon finding after transanal surgery or transanal endoscopic microsurgery procedures, particularly when performed for T2 lesions with mucinous or poor differentiation [23]. These occurrences direct many coloproctologists toward intraoperative cytocidal rectal washout before colorectal anastomosis [24]. Isolated colorectal anastomotic recurrence has a better prognosis than extraluminal recurrence, and detection of such cases in the absence of extraluminal disease is an indication for radical curative repeat resectional surgery. Occasionally, the “recurrent tumor” may be noted to be near but not at the anastomotic line. Such cases, coupled with no recurrence elsewhere as seen on extensive imaging, may indicate a metachronous cancer due to field change, and careful colonoscopy is, therefore, vital in deciding the most appropriate resection and extent of resection to be undertaken.
Ileal Pouchitis
Endoscopy is one of many available tools used to evaluate structural abnormalities of these pouches, including pouch inlet and outlet strictures, pouch fistulas, and sinuses and leaks. The accuracy of endoscopy is enhanced by combining it with other methods of imaging, including CT enterography, gastrograffin enemas, and MRI [25]. A specific complication is a leak from the blind end of the “J” pouch, which may require ultrasound- or CT-guided drainage and catheter placement. Over time, when repeated radiology is needed in some patients, MRI will be favored over the radiation exposure of repeated CT. In this respect, CT enterography seems to have the lowest accuracy for the diagnosis of small-bowel strictures, inlet strictures, or both, whereas MRI has the lowest accuracy for pouch sinuses; combining these modalities with pouch endoscopy increases the overall diagnostic accuracy in these conditions. The main advantage of endoscopy over the other modalities is the ability to obtain tissue biopsies, where histological analysis may reveal a diagnosis of Crohn’s disease after a previous diagnosis of ulcerative colitis or show the presence of dysplastic mucosa after previous surgery for familial adenomatous polyposis. Endoscopic mucosal surveillance detects and grades pouchitis and monitors treatment, which has implications for the diagnosis of pouch failure and will help determine whether a pouch salvage procedure or pouch excision should be undertaken.
Anastomotic Stricture
Anastomotic stricture after colorectal or coloanal anastomoses occurs in up to 30 % of patients [26–28]. Direct visualization with endoscopy allows the surgeon to determine the location, nature, length, and extent of the stricture as well as the opportunity for biopsy. Asymptomatic strictures discovered incidentally during radiological examination or endoscopically may not need intervention; a narrowed anastomosis in the early postoperative period normally dilates naturally with time. Symptomatic strictures, however, need further evaluation and treatment, as indicated by the symptoms or cause. Endoscopy permits balloon dilatation, which has been reported to be successful in up to 90–100 % of patients (Figs. 5.3, 5.4, and 5.5) [27, 29, 30], which may be supplemented by deployment of a self-expanding metallic stent. Recently, Forshaw et al. [31] have reported successful outcomes with endoscopic transanal stricture resection for the management of high-grade anastomotic strictures; there is a short length of hospital stay, although there is an incidence of reoperation for bleeding and reported microperforations, with technical difficulties with its use if the anastomosis is acutely angulated. Several endoscopic electrocision techniques have been described, also using a papillotomy or hook knife in combination with fluoroscopic identification of the proximal bowel, particularly if the original anastomosis is an end-to-side configuration [32–35]. In this regard, some techniques have been associated with a relatively high rate of restricturing [32].
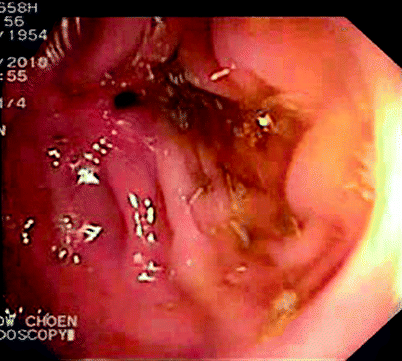
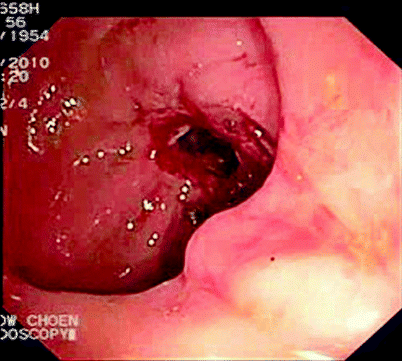
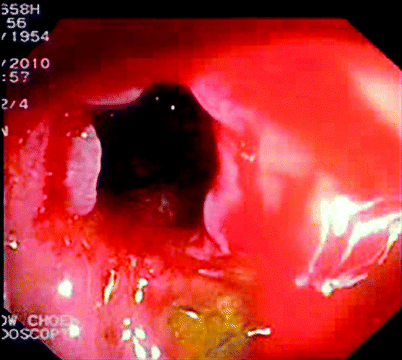

Fig. 5.3
Stricture seen on colonoscopy

Fig. 5.4
Stricture after attempted passage of colonoscope

Fig. 5.5
Stricture after endoscopic dilatation
Further alternatives have included endoscopic steroid injection of Crohn’s strictures, although single treatments do not reduce the time to repeat dilatation, and at least two injections are required in most cases [36]. More recently, biodegradable stents have been used, but there is no clear relationship between the number of dilatations required in combination with stenting and the incidence of symptomatic stricture recurrence [29, 37, 38]. In these patients, there is a greater self-reported incidence of gastrointestinal symptoms in patients undergoing dilatation, including frequent bowel movements, bloating, abdominal pain, and constipation or diarrhea, with an impaired reported health-related quality of life that is somewhat independent of the delay between stricture dilatation and the time of assessment [39]. This probably is representative of a disparity between radiological, endoscopic, and patient-related clinical symptoms in the definition of stricture severity and length.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







