Fig. 17.1
Tumour resection: a Preoperative breast markings. b A 220-g specimen attached to ‘access tunnel’ tissue, showing the resulting resection defect and lateral incision used to perform the procedure. c Bed biopsy material sent for intraoperative frozen section. d ‘Re-excision specimens’ inked in situ with methylene blue to identify the surface adjacent to the cavity
17.3.3 Incisions and Raising the Flap
The incision depends on whether volume is being replaced in the immediate or delayed setting and which type of flap is planned. In the delayed setting, reopening the previous skin incision on the breast often reveals a skin deficit. The wound may gape, demonstrating that skin replacement is required to allow the remaining breast tissue to return to its presurgical position. This may be the case even if no skin was excised at the time of breast-conserving surgery. Despite being an “apparent” skin deficit as a result of scarring and radiotherapy rather than a real deficit, it will need correction to optimise the result.
When the tumour is excised immediately before volume replacement, the breast skin is mobilised in the oncoplastic plane, over and around the tumour. The breast is then mobilised off pectoralis major muscle. The tumour is excised with generous margins and in continuity with lateral tissue to form the access tunnel as required. Bed biopsies are performed and the material removed is sent for frozen section, and a full cavity re-excision is undertaken to maximise the chance of clear margins (Fig. 17.1) [24]. Alternatively, a “delayed-immediate” reconstruction can be undertaken 1 week after tumour excision and when final histopathology results are available [29]. This is a particularly useful option after neoadjuvant chemotherapy when frozen-section analysis is more likely to be falsely negative.
An immediate LD miniflap is best performed via a cosmetically discreet “lazy S” incision in the anterior axillary line, providing access to the breast for tumour excision and the back for raising the LD flap [30]. The LD flap is raised in the plane just beneath the fascia, sparing the subcutaneous fat but taking a layer of fat over the muscle, which contributes to the flap volume (Fig. 17.2). Division of the entire fascial attachment of the LD muscle to teres major muscle, all serratus anterior muscle branches and the tendon of the LD muscle allows full transposition of the flap into the resection defect (Fig. 17.3). This is particularly important when reconstructing more remote defects in the medial or lower pole of the breast. Finally, the tendon needs to be secured to pectoralis major muscle to prevent unintentional tension on the pedicle, before the flap is modelled and sutured into the resection defect (Fig. 17.4). When harvesting the flap it is best to overestimate the volume required to allow for muscle atrophy over time. As a result, the volume of the reconstructed breast should be larger than that of the opposite breast at the end of the procedure, and a good cosmetic result can be anticipated if these key steps are observed (Fig. 17.5).
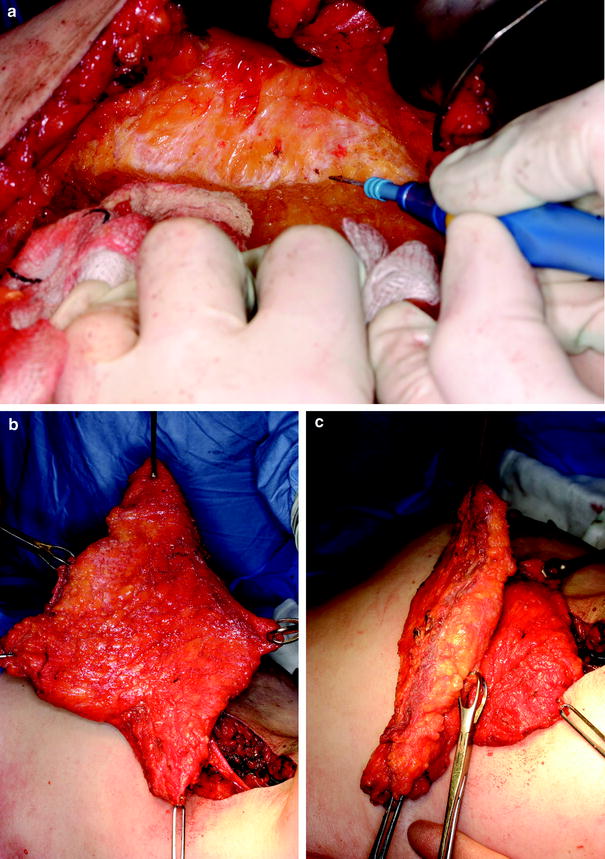
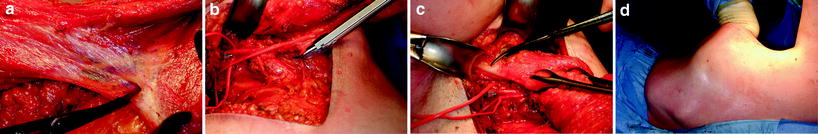
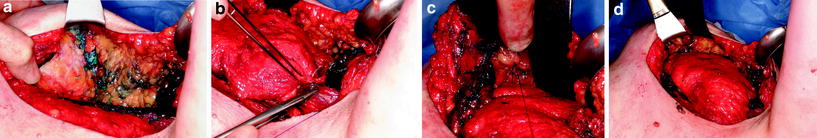
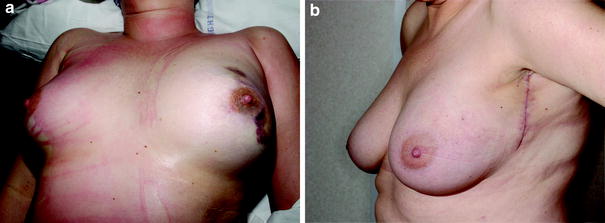

Fig. 17.2
Harvesting the latissimus dorsi (LD) miniflap. a Dissection of the superficial surface of the flap in a plane immediately under Scarpa’s fascia (the deep fascia). b The layer of fat on the superficial surface of the flap harvested as a result of dissecting in this plane. c View of the divided distal end of the flap, showing the layer of superficial fat, which is thicker than the muscle itself at this level

Fig. 17.3
Division of all LD miniflap attachments and the resulting donor defect. a Division of the well-developed fascia between LD muscle (top left) and teres major muscle (bottom right), dissecting in a cranial direction. The thoracodorsal vessels lie immediately deep to this unnamed fascial layer. b Clip ligation of a serratus anterior muscle branch in preparation for division of the vessels. c Protecting the subscapular vessels with a sling during division of the LD tendon. d The assistant’s hand outlines the extent of the LD donor defect following flap harvest

Fig. 17.4
Reconstruction of the resection defect. a Lateral view of the walls of the resection defect. b Suturing the tendon of the LD miniflap to the lateral border of pectoralis major muscle. c Suturing folded distal edge of the flap onto the medial cavity wall. d Appearance of the flap at end of the procedure after it has been sutured into the resection defect

Fig. 17.5
Postoperative appearance. a Appearance before extubation, showing over-replacement of the resected volume to allow for subsequent volume loss. b Appearance at 6 weeks, showing short ‘lazy-S’ lateral scar and natural breast shape
Perforator flap incisions are planned to incorporate the best perforators, and with a view to minimising the cosmetic defect. This is usually an ellipse in the bra line, sited with the anterior tip at the lateral border of the inframammary fold. Alternatively it may be oriented vertically, sparing the medial back, if the patient wishes. TDAP flaps are raised in a plane above or just below the deep fascia and the perforator is identified and dissected through the muscle and up to the thoracodorsal artery itself until the required pedicle length has been achieved (for details see Hamdi et al. [23]). Perforators from the descending branch of the thoracodorsal artery are preferred over perforators from the transverse branch but the inconsistent perforator size, quality, quantity and location means dissection must be painstaking, and is often reported as tedious [23, 28, 31]. If the perforator is less than 0.5 mm in diameter, the flap is at risk of failure, so conversion to a muscle-sparing LD flap is advised. The flap is brought through the muscle and placed in the defect, such that the anterior border lies medially or is rotated to lie inferiorly.
LICAP flaps are based on the perforators arising from the costal segment of the intercostal artery. In a cadaveric dissection study, Hamdi et al. [19] showed a variable number of intercostal perforators and a dominant perforator in 92 % of cases: these lay, on average 3.5 cm from the anterior border of the LD muscle. If the pedicle is long enough, the flap can be rotated through 180° [19, 21]. If the perforator is eccentric within the area of the flap, this rotation may allow significantly greater reach.
All flaps are partially or totally de-epithelialised, depending on whether skin is required to replace a deficit. If the flap is totally subcutaneous, Doppler monitoring cannot be undertaken.
17.4 Outcome
The literature on volume replacement comprises mainly single-institution series, i.e. level 3 evidence. There is a lack of objective outcome reporting, very few comparative studies and it is likely that publication bias exists. It is not clear whether volume replacement techniques are being widely used by surgeons other than the recognised experts such as Hamdi, Rainsbury and Munhoz. Those achieving less successful outcomes are less likely to report their results.
17.4.1 Oncological Outcomes
Although the literature on “oncoplastic surgery” as a whole is expanding rapidly, it remains very heterogeneous in the indications for surgery, the techniques used and the duration of follow-up. Overall, oncoplastic breast-conserving surgery has a local recurrence rate approximately equivalent to that of standard breast surgery, which may be because of the balance between allowing wider excision for some tumours while being used in patients with more extensive disease [32–35].
Tables 17.1 and 17.2 summarise the limited literature on local recurrence after volume replacement with distant flaps. For LD miniflap series, the local recurrence rates range from 0 to 5 % with a stated follow up of 24–54 months. It is striking that most reports on perforator flap surgery focus on the techniques of surgical reconstruction This may reflect the interests of the population of surgeons undertaking the different forms of reconstruction, with more breast/general surgeons doing LD miniflaps and plastic surgeons doing perforator flaps. Alternatively, it may simply be because perforator flaps have been used in fewer patients and more recently so that follow-up data are only now becoming mature enough for scrutiny [21]. Finally, it is reasonable to assume that the oncological decision-making with regard to tumour excision is dissociated from the method used to fill the defect, so the local recurrence rate should not differ according to reconstructive technique used. However, Rietjens et al. [35] report that LD volume replacement was used for cases with a large defect and that larger tumours had a higher recurrence rate, so it is possible that over time a trend will emerge.
Table 17.1
Case series of latissimus dorsi miniflap (LDMF) volume replacement
Authors | Flap | Number | Tumour size | WLE weight/volume | LR and follow-up | Cosmesis | Complications |
|---|---|---|---|---|---|---|---|
Noguchi et al. [14] | LDMF | 5 | 4/5 good cosmesis by moire topography | ||||
Raja et al. [30] | LDMF | 20 | 25 mm | 57 % > 150 g | Cosmetic failure uncommon (10 % vs. WLE 34 %) | ||
Kat et al. [36] | LDMF | 30 | Two minor wound infection, six seromas | ||||
Dixon et al. [29] | LDMF | 25 | Median 94 g | Similar to WLE | 21 seromas, no other major morbidity | ||
Gendy et al. [22] | LDMF | 49 | 22 mm | 2 LR at 53 months but had not had RT | Significantly better than for SSM | 6 % required further surgery. One brachial plexopathy | |
Losken et al. [37] | LDMF | 39 | 5 % at 44 months | ||||
Nano et al. [38] | LDMF | 18 | Median 33 mm | 130 g | 0 at 24 months | 17/18 satisfied (1 required mastectomy) | 14 seromas, no major complications |
Munhoz et al. [39] | LDMF | 48 | 44 % > 2 cm | Flap complications in 7, donor site in 12 | |||
Naguib [40] | LDMF | 29 | Median 5.2 cm | 219 cm3 | 69 % cosmetically satisfactory | Persistent seroma 52 %. No sepsis or flap viability problems | |
Navin et al. [41] | LDMF | 51 | 20 mm | 217 g | None at mean of 33 months | 86 % of respondents satisfied | One flap necrosis |
Rusby et al. [24] | LDMF | 110 | 34 mm | 207 g | 1 at median of 41.4 months | Three infection/wound problems | |
Hernanz et al. [42] | LDMF | 41 | 22 mm | Median 167 cm3 | 1/41 (2.4 %) at 54 months | 65 % satisfactory |
Table 17.2
Case series of other pedicled flaps in reconstruction after breast-conserving surgery
Authors | Flap | Number | Tumour size | WLE weight/volume | LR and follow-up | Cosmesis | Complications |
|---|---|---|---|---|---|---|---|
Hamdi et al. [18] | TDAP ICAP | 18 3 | Two partial flap necrosis | ||||
Hamdi et al. [23] | TDAP to various sites 73 immediate partial reconstruction, 5 delayed |









