Figure 13-1 Third-degree burn. A: The epidermis shows full-thickness eosinophilic necrosis resulting in bullous disassociation from the dermis and incipient demotion. This bulla formation can be seen in second- and third-degree burns. The collagen shows homogenized necrosis, with loss of distinction between collagen bundles. The fibroblasts are nonatypical, differentiating this from chronic radiation injury. B: There is pandermal eosinophilic homogenized alteration collagen, with necrosis of an eccrine gland. The epidermis shows bulla formation. C: Extension of the injury involving the pilosebaceous units differentiates a third-degree from a superficial second-degree burn. Burn injury to the pilosebaceous unit results in eosinophilic necrosis of the epithelium and elongation of the nuclei. The surrounding stroma shows coagulation of the collagen and vascular thrombosis. D: Eosinophilic alteration of an injured eccrine gland differentiates third- from second- and first-degree burns.
Third-degree burns: Full-thickness coagulation necrosis of the epidermis and dermis, including the appendages, is seen (Fig. 13-1). The resultant scar is characterized by hyalinized collagen and an absence of adnexal structures (Fig. 13-2).
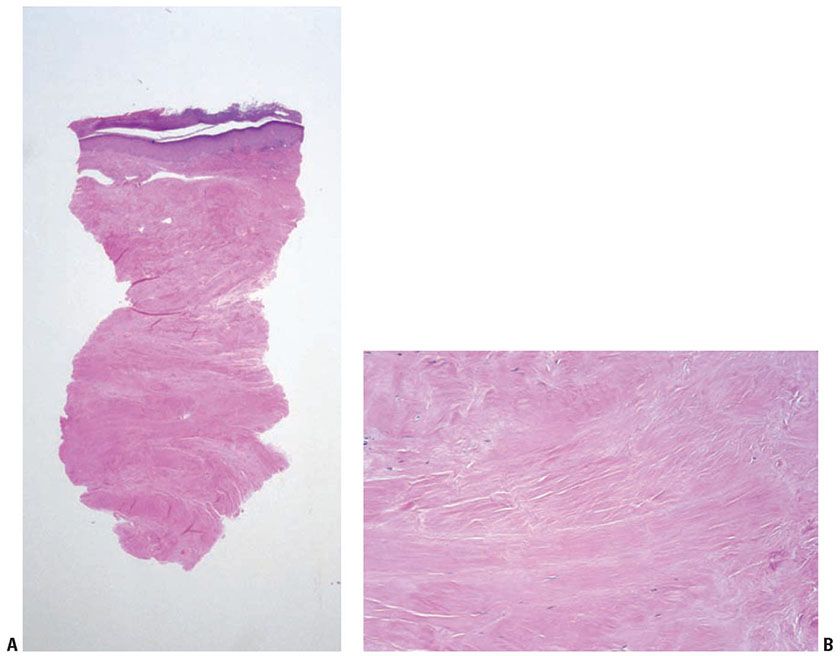
Figure 13-2 Chronic burn scar. A: The dermis and subcutis are diffusely sclerotic and homogenized, with complete absence of adnexal structures, including arrector pili muscles, differentiating this from radiation injury and scleroderma/morphea. The absence of vascular proliferation and presence of homogenized thickening of the collagen differentiate a chronic burn from a scar from other reparative causes. B: The sclerotic collagen is nearly devoid of fibroblasts, in particular atypical ones, differentiating this from chronic radiation injury.
Chemical burns: Chemical burns may vary, depending on the substance. Dermal and adnexal necrosis similar to thermal burns may be seen. Histopathology of sulfur mustard shows four patterns:
1. Interface dermatitis, with or without necrosis, similar to toxic epidermal necrolysis or erythema multiforme, with hyperpigmentation of the epidermal roof.
2. Spongiotic dermatitis, similar to acute contact dermatitis. Bulla formation is often at the dermal–epidermal junction, with or without acantholytic cells. The epidermal roof shows dysmaturation with cellular atypia.
3. Hyperpigmentation of the basal layer of the epidermis with dermal melanophages.
4. Dermal alteration, including coagulation necrosis, low-grade fibrosis with sclerodermoid changes, telangiectases, and perivascular inflammation, without vasculitis (3).
Differential Diagnosis. Radiation dermatitis is differentiated from burn scars by the presence of atypical radiation fibroblasts in the former. Complete absence of the adnexal structures favors a burn scar because the eccrine glands may be spared with radiation. In morphea/scleroderma, there is preservation of eccrine coils, and a lymphoplasmacellular infiltrate may be seen.
Principles of Management. Management of burns is dependent on the extent of injury—the degree of burn (first, second, third), the body surface area involved, and the anatomic region affected. Superficial burns are improved by application of ice, cold water, and/or cold compresses. Prevention of infection is of paramount importance, especially for patients affected by deeper burns. Topical antimicrobial agents are commonly used for this reason, including silver sulfadiazine. Excision of burn wounds that extend into the deep reticular dermis and subcutaneous fat may be performed to prevent wound infection and limit morbidity. Careful attention should be given to the cardiopulmonary status of a patient with extensive injury (greater than 10% to 15% body surface area) and/or deep burn wounds, given the risk of insensible water losses and sepsis as a result of compromised skin barrier function. Such patients may be best managed in the setting of a burn center.
Burn Scar-Associated Malignancy
Chronic burn scars are subject to the development of dystrophic calcinosis cutis (11), and carcinomas, especially basal cell carcinomas, may arise acutely within 2 to 3 months after the injury (1). Delayed carcinomas arise after a latency that ranges from 10 to 70 years, with an average of approximately 30 years. There may be a shorter lag time (19 years) if there is poor wound care (12). The tumors most often arise on the extremities but may also arise on the head and trunk. Burn scar-associated cancers include squamous more often than basal cell carcinomas, but malignant melanoma (13), angiosarcoma (14), and anaplastic large cell lymphoma (15,16) may be seen. Squamous cell carcinomas are more often seen with scalding burns, whereas basal cell carcinomas are associated with burns from flames. Welders are also at risk for getting both basal and squamous cell carcinomas due to chronic exposure to both high temperatures and nonsolar ultraviolet (UV) radiation (17,18). The occurrence of cancer developing in burn scars was noted in 1860 by Heurteux, and trauma-induced carcinomas were described by Marjolin in 1828; these cancers are more aggressive than their sun-induced counterparts. Metastases may occur in up to approximately one third of the cases. Recurrence is also higher in cancers due to these chronic ulcers. The carcinomas arise from the edges of wounds or from epithelial remnants entrapped in the scar; thus, they may not occur as frequently if there has been grafting (1).
Acute Ultraviolet Burn
Mild UV-induced burns are commonly referred to as “sunburns.” The resulting erythema is characterized by the presence of scattered apoptotic keratinocytes on histopathology, termed “sunburn cells.” Variable subepidermal edema is seen. Clinical blistering may result from severe burns due to full-thickness epidermal necrosis and/or dermal edema (Fig. 13-3). The preservation of appendages allows for epidermal regeneration.
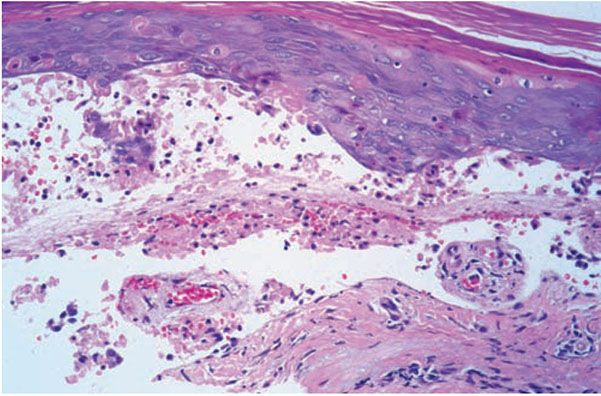
Figure 13-3 Acute UV radiation burn. This is an example of a UVA-induced burn showing full-thickness dyskeratotic keratinocytes throughout all layers of the epidermis, with subepidermal blister formation.
Ultraviolet Recall
Clinical Summary. UV recall refers to the phenomenon in which a medication induces an acute cutaneous eruption restricted to the area of previous sunburn or UV light therapy (19). Other names for this phenomenon include sunburn reactivation, sunburn recall, photo recall, and photodermatitis reactivation. UV recall is seen after methotrexate use, but it can also be associated with other chemotherapeutic agents, targeted therapy agents (including sorafenib), and antibiotics, such as ampicillin and trimethoprim–sulfamethoxazole (20). Two clinical presentations may be seen, depending on whether the UV recall reaction occurs days after UV exposure, as is seen with methotrexate, or weeks to months later, as is typically seen with antibiotics. Methotrexate-induced recall phenomenon generally occurs if methotrexate is given 1 to 5 days after sunburn occurs, but not if the burn is concomitant or much earlier. This UV recall tends to be associated with pain and burning, with an erythematous to violaceous vesiculobullous eruption (19), and in most cases it is more severe than the initial sunburn. UV recall reactions associated with antibiotics (21,22) differ from the methotrexate reaction. These tend to occur at a later interval—weeks to months from the sunburn—with a less severe, pruritic macular, papular; or morbilliform-like drug reaction (22). The UV reaction with either presentation presents within a few hours or days of the medication intake. Rechallenge may occur at a shorter interval than the initial reaction and with a more severe blistering reaction (23). It has been proposed that the methotrexate-induced reaction be considered a UV enhancement reaction and those associated with a delayed interval of UV exposure be considered UV reactivation (19).
Histopathology. Histologic features of UV recall are not well documented because the diagnosis is usually made clinically. A review of the literature described several histologic features, most of which indicate a component of interface or cytotoxic dermatitis, characterized by hydropic degeneration, dyskeratotic or apoptotic keratinocytes, dysmaturation, or acanthosis. There is also a variable perivascular lymphocytic infiltrate in the papillary dermis, sometimes with erythrocyte extravasation. Neutrophils have been described. Clinical blisters have been associated with extensive epidermal necrosis with intraepidermal bullae formation (19).
Principles of Management. Treatment of UV recall is supportive. The eruption will spontaneously resolve over time. Topical steroids and emollients may provide symptomatic relief.
Erythema Ab Igne
Clinical Summary. Erythema ab igne is a clinically distinctive skin finding, which manifests as reticulated, dusky red to brown patches (Fig. 13-4). Telangiectases may be seen within the patches. Erythema ab igne commonly manifests following repetitive, direct exposure of the skin to a heat source. It was traditionally found to occur on the shins, buttocks, and back as a result of exposure to open hearths or stoves, steam heaters, and hot water bottles (24). Laptop computers have been more recently reported to induce erythema ab igne, often on the thighs (25). Heated car seats and recliners are also newer associations (26–28). Patients with altered mental status may be at increased risk of erythema ab igne, given decreased awareness of prolonged exposure to heat sources, including heating blankets (29).
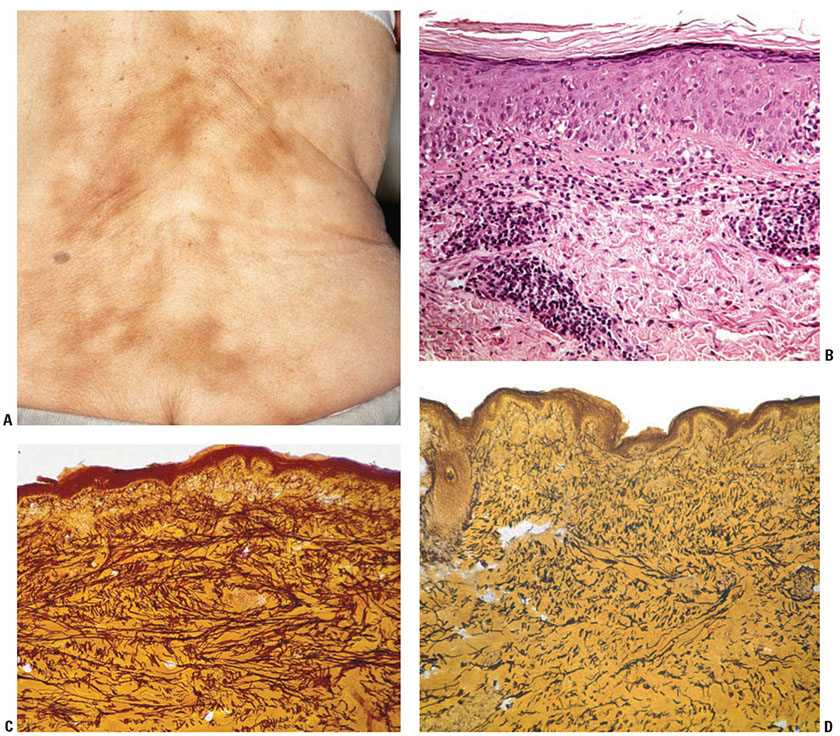
Figure 13-4 Erythema ab igne. A: Clinically, reticulated brown patches are seen in erythema ab igne. This may present on the lower back, where there has been frequent use of a heating pad. B: Erythema ab igne has variable histologic features. This hematoxylin and eosin-stained biopsy shows a perivascular lymphocytic infiltrate in the superficial dermis that focally disrupts the dermal–epidermal interface. There is no discernible elastic fiber abnormality noted on routine staining. The absence of clumped basophilic elastic tissue differentiates erythema ab igne from solar elastosis. C: Staining for elastic tissue highlights the increased amount of elastic tissue in comparison to uninvolved skin (see D), characteristic of erythema ab igne. D: Elastic staining of uninvolved skin, showing a normal distribution of elastic fibers, as compared with C. (Photographs courtesy of Waine Johnson, MD.)
In addition to pigmentary alternation, secondary clinical findings may occur in erythema ab igne. Specifically, formation of bullae has been observed (30,31). Thermal keratoses (which histologically show partial-thickness epidermal atypia, akin to actinic keratoses) and squamous cell carcinomas may arise in areas of erythema ab igne (32). Merkel cell carcinoma, poorly differentiated carcinoma, and cutaneous marginal zone lymphoma have also been described to occur in erythema ab igne (33–36).
Histopathology. The histopathologic findings of erythema ab igne are often subtle, and may be nonspecific in the absence of correlative clinical information. The epidermis may be normal or atrophic, with effacement of the epidermal rete. Interface dermatitis, featuring mild basovacuolar change, dyskeratotic keratinocytes, and a squamatized basal epidermal layer, is observed. There is mild spongiosis and prominent papillary dermal edema (37,38). Pigment incontinence is frequently seen, and consists of melanin-laden macrophages as well as extracellular melanin granules (39). Hemosiderin deposition has been described, although this may be attributed to the fact that many biopsies of erythema ab igne are taken from the lower legs, and are more likely to display extravasated red blood cells (37). Dilated small vascular channels are present in the superficial dermis, sometimes engorged with red blood cells. Enlarged, atypical endothelial cells have been observed, demonstrating hyperchromatic, irregularly shaped nuclei (38). A mixed angiocentric infiltrate of variable intensity is observed in the superficial dermis, composed of lymphocytes, macrophages, neutrophils, plasma cells, and mast cells. Increased numbers of elastic fibers are seen in biopsies of erythema ab igne, highlighted by elastic stains (24) (Fig. 13-4), and fragmentation of elastic fibers may occur (31). Alcian blue stains detect increased hyaluronic acid in the papillary and reticular dermis (24).
Thermal keratoses showing basal keratinocytic atypia and squamous cell carcinoma in situ have been documented to arise within erythema ab igne, often occurring on the lower leg (32,40). A case of cutaneous marginal zone lymphoma, demonstrating a dermal infiltrate consisting of a diffuse, monotonous proliferation of CD20+ CD5− CD10− lymphoid cells with round to slightly irregular nuclei, has been reported (36). In bullous erythema ab igne, there is a subepidermal plane of separation associated with epidermal atrophy, rete effacement, and hydropic degeneration of basal cells with melanophages, consistent with a lichenoid tissue reaction (31). Cases of bullous erythema ab igne have been attributed to coexisting lichen planus (41), but may simply represent a manifestation of intrinsic blistering. Direct immunofluorescence on these cases are either negative or show a pattern of immunoglobulin M staining in the papillary dermis, consistent with colloid bodies. Ultrastructural investigations of erythema ab igne have been reported (42), and show changes consistent with apoptosis of the basal keratinocytes, functional activation of melanocytes, and elastic fiber alterations indistinguishable from actinic elastosis due to chronic UV radiation. The keratinocyte change appears to represent heat-related damage and not a preneoplastic alteration. The vessels are dilated and increased in number. This may rarely present as cutaneous reactive angiomatosis, mimicking a malignant vascular neoplasm or diffuse dermal angiomatosis (43).
Differential Diagnosis. The elastic tissue alteration induced by UV radiation in solar elastosis is apparent, on hematoxylin and eosin staining, as a solid and homogenized material, as opposed to erythema ab igne, which, on routine staining, shows no perceptible elastic tissue changes. Erythema chronicum perstans may also show subtle interface dermatitis with pigment dropout, but is distinguished from erythema ab igne by the clinical presentation and the absence of increased elastic fibers. A hypersensitivity reaction with interface changes may show some overlapping features with erythema ab igne, but the clinical history should prompt elastic tissue staining, differentiating the two entities.
Principles of Management. Withdrawal of the offending heat source is essential to prevent worsening or recurrence of erythema ab igne. The pigmentary changes will resolve slowly over time. Treatment with Nd-Yag lasers has been reported to be beneficial for pigmentary alteration (44).
RADIATION-INDUCED SKIN ALTERATIONS
Radiation is frequently administered therapeutically for diagnostic purposes or for the treatment of primary and metastatic malignancies. In the past, it had been used to treat inflammatory conditions of the skin, including acne and eczema, although this is no longer common practice. Radiation-induced dermatoses are commonly seen, and include acute and chronic radiation dermatitis, radiation recall dermatitis, and secondary cutaneous neoplasms. Radiation dermatitis is a common inflammatory skin finding that is the direct result of exposure to radiation, and is dose dependent. The injury is the result of biochemical changes in X-ray-penetrated cells without a concomitant rise in temperature. Changes of radiation dermatitis appear at the portal of radiation entry or exit. In addition to the dose of irradiation, additional host factors play a role in the development of radiation dermatitis. Patients with the inherited condition ataxia telangiectasia (A-T) syndrome display greater than normal radiation sensitivity (45); other DNA repair disorders characterized by exaggerated responses to radiation include Fanconi anemia, Nijmegen breakage syndrome, and DNA ligase IV deficiency (46). Acute and chronic radiation toxicity has been reported in individuals with rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, scleroderma, diabetes mellitus, and hyperthyroidism (47). Concomitant use of radiation-sensitizing medications, such as doxorubicin and taxane antineoplastic agents, may predispose an individual to increased acute toxicity reactions to radiation (48). Over a longer period of time, radiation may induce neoplasms, which is a dose-independent effect. Fluoroscopically guided procedures may cause radiation dermatitis in areas of skin not directly in the radiation port, usually on the back (49). Radiation recall dermatitis refers to the induction of an inflammatory reaction limited to a site of a quiescent radiation field by administration of a medication. Certain inflammatory conditions may be exacerbated by radiation, for example, vitiligo (50), lichen planus (51), bullous pemphigoid (52), and graft-versus-host disease (53).
Acute, Subacute, and Chronic Radiation Dermatitis
Clinical Summary. Radiation dermatitis can be classified into acute, subacute, and chronic phases. Acute radiation dermatitis presents within 90 days of radiation exposure (Fig. 13-5) (54). A transient erythema may appear within 24 hours of the radiation insult and resolve within several hours to days. A second, more persistent erythema is then apparent about 10 to 14 days after the initial exposure. In addition to erythema, other cutaneous changes, including desquamation, alopecia, xerosis, dyschromia, blistering, epidermal and dermal atrophy and necrosis, erosion and ulceration, may be seen in acute radiation dermatitis. The phases of acute radiation dermatitis have been outlined in detail, and criteria have been put forth by the National Cancer Institute (54). Grade 1 radiation dermatitis includes generalized erythema with dry desquamation, which may be accompanied by pruritus and dyspigmentation. This may be concentrated in the follicles, resulting in alopecia due to injury to the pilosebaceous glands. Grade 2 radiation dermatitis typically occurs in the skin folds 4 to 5 weeks after treatment of doses 40 Gy or greater. There is persistent tender edematous erythema, which may progress to bullae or focal epidermal necrosis, with fibrinous exudates, termed moist desquamation. Superinfection may occur at this stage. Grade 3 shows an increased area of moist desquamation compared to Grade 2, extending beyond the skin folds. Grade 4 is characterized by ulceration, necrosis, and hemorrhage. Unresolved acute radiation results in chronic ulceration, fibrosis, and/or necrosis of the deeper structures, which is termed “consequential late” radiation dermatitis. Subacute radiation dermatitis occurs weeks to months after radiation exposure, but is otherwise not well-characterized clinically (55).
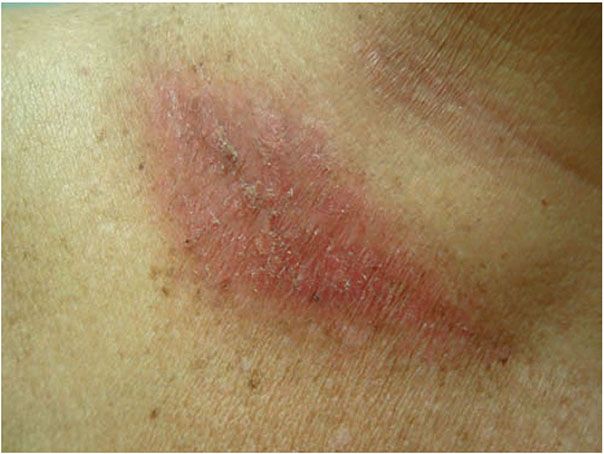
Figure 13-5 Acute radiation dermatitis. Clinically, this presents as a scaly erythematous plaque, with geographic borders defined by the radiation field.
Changes of chronic radiation dermatitis are noted months to years after the original insult, but often represent progression of acute and subacute radiation dermatitis and therefore may be evident earlier. Characteristic findings of chronic radiation dermatitis include epidermal atrophy, xerosis, hypo- and hyperpigmentation, alopecia, anhidrosis, poikiloderma, hyperkeratosis, telangiectases, and fibrosis of the dermis and subcutis are seen. Late-onset dermal necrosis and changes of chronic radiation dermatitis with or without prior acute radiation changes may be seen years after the initial radiation. Nonhealing ulcers may be seen. Deep megavoltage radiation for noncutaneous malignancies may result in subcutaneous sclerosis that may extend into the underlying skeletal muscle (56).
Histopathology. Histopathologic changes of radiation dermatitis vary with the stage of the lesion sampled (49).
Acute radiation dermatitis shows spongiosis and scattered dyskeratotic keratinocytes in the epidermis. Depending on the dose of radiation, epidermal necrosis with blister formation and sloughing of the epidermis—which corresponds to the clinical findings of “moist desquamation”—may be seen. Hyperkeratosis is often observed. In the dermis, frequent findings include dermal edema, endothelial cell swelling, vasodilation, red blood cell extravasation, and fibrin thrombi of vessels. Variable inflammation is noted throughout the dermis, with involvement of the epidermis. Acute necrotic changes similar to those seen in the epidermis may also occur in the adnexal epithelium, eventuating in absence of pilosebaceous structures. Complete destruction of eccrine glands is infrequently observed. In severe radiation injury, necrosis of the epidermis and dermis results in persistent ulceration.
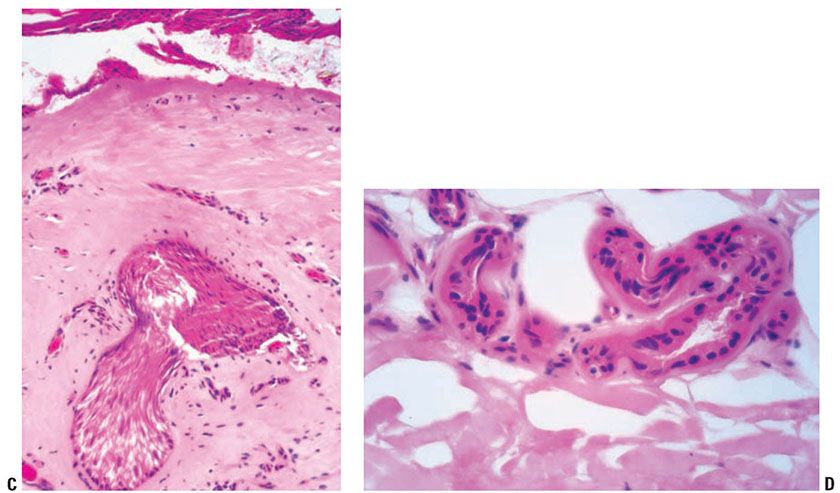
Late-stage or chronic radiation dermatitis is characterized by eosinophilic homogenized thickening of the dermal collagen, large atypical “radiation fibroblasts,” lack of pilosebaceous units, and vascular changes (Fig. 13-6) (57). The superficial dermis shows thin-walled, markedly dilated vascular channels set within an edematous, homogenized stroma, while the deep vessels show fibrous thickening, sometimes with luminal obliteration and recanalization. The overlying epidermis may be atrophic and hyperkeratotic. Features of an interface reaction are sometimes present (Fig. 13-6). A maturation disorder with nuclear atypia and individual cell keratinization may be seen. Juxtaposed to atrophic areas may be epidermal hyperplasia that can extend downward and encase the telangiectatic vessels.
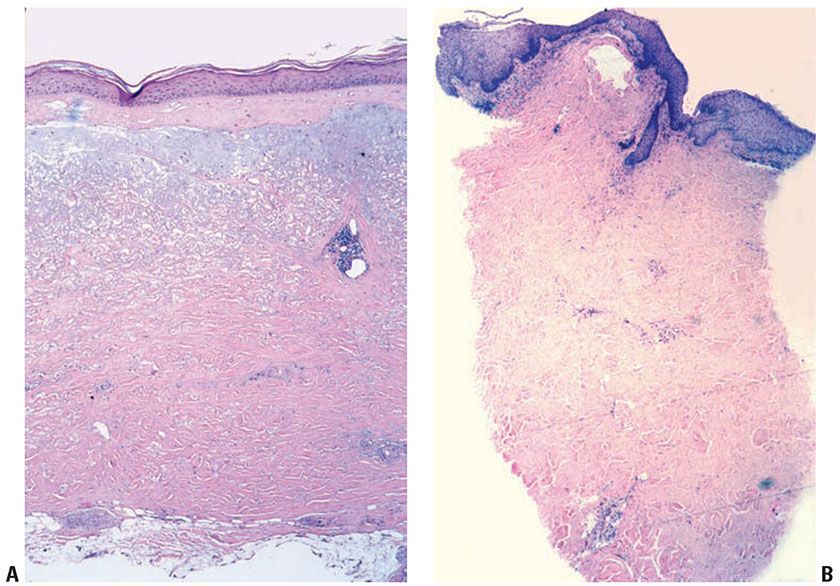
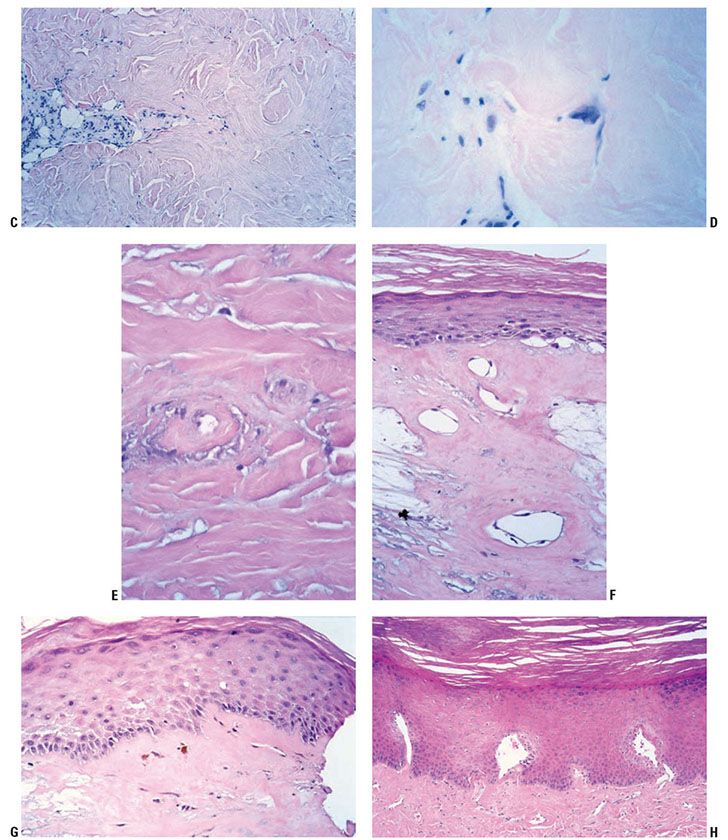
Figure 13-6 Late-stage radiation injury. A: This biopsy shows features of chronic radiation dermatitis, including epidermal atrophy, pandermal collagen sclerosis with mild lymphocytic inflammation, and absence of hair follicles. B: A rectangular, “squared-off” silhouette due to the sclerotic collagen alteration. This biopsy also demonstrates the variation of epidermal atrophy and hyperplasia that can be seen in radiation dermatitis. C: There is sclerotic collagen bundle thickening, with compression of eccrine glands. Preservation of eccrine glands differentiates this from a burn scar. D: Large, bizarre, and atypical radiation fibroblasts amid the thickened collagen bundles differentiate radiation dermatitis from morphea and third-degree burn scars. These are sparsely scattered, in contrast to atypical cells of a sarcoma. E: Sclerosis of endothelial cells is seen amid thickened sclerotic collagen bundles. F: There is telangiectasia of the upper dermal vessels, with epidermal atrophy and sclerosis of papillary dermal collagen. G: The epidermis shows features of interface dermatitis, with liquefaction of the basal layer, rete effacement, and pigment dropout. H: The epidermis may show hyperplasia, incorporating the underlying telangiectatic vessels between the rete.
The origin of the radiation fibroblast is not known. Immunohistochemical studies have shown cytoplasmic staining of radiation fibroblasts with factor XIIIa antibody, and only focal CD34 positivity in some cells (58). However, factor XIIIa staining is not consistently observed in all studies. The radiation fibroblasts have been shown to be negative for HHF-35 (muscle-specific actin), and Ki-67, and p53 (59).
Subacute radiodermatitis shows overlapping features of acute and chronic radiation dermatitis. There is an interface dermatitis, with basal vacuolization and keratinocyte dyskeratosis (Fig. 13-7) (60). The presence of satellite cell necrosis, characterized by close apposition of dyskeratotic keratinocytes and CD8+ TIA-1-positive T lymphocytes, suggests that cytotoxic lymphocyte-mediated apoptosis is involved in the pathogenesis of subacute radiation dermatitis (55).
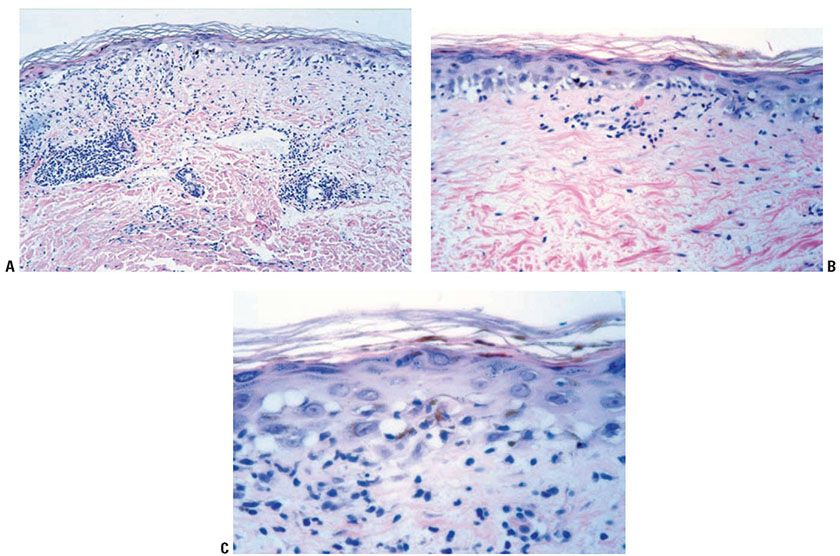
Figure 13-7 Subacute radiation dermatitis. A: In subacute radiation dermatitis, there is an inflammatory infiltrate involving the epidermis, papillary dermis, and vessels, without sclerotic collagen alteration. The epidermis is atrophic due to interface dermatitis. B:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








