The practice of plastic surgery is a unique mixture of art and science, and both must be carefully balanced to provide the best possible care for patients. To do that, clinicians should be practicing evidence-based medicine. This article discusses the prevalence and risks associated with deep vein thrombosis and the reasons and options for its possible chemoprophylaxis. Until evidence-based medicine best-practice recommendations can be developed, it would be prudent for clinicians to empirically select and consistently apply a risk stratification system and prophylaxis regimen of their choice for the benefit of their patients.
Key points
- •
The practice of plastic surgery is a unique mixture of art and science, and both must be carefully balanced to provide the best possible care for patients.
- •
Clinicians should ideally be practicing evidence-based medicine to help clinicians predict whether a treatment will do more good than harm.
- •
Until evidence-based medicine best-practice recommendations can be developed, it would be prudent for clinicians to empirically select, and consistently apply, a risk stratification system and prophylaxis regimen of their choice for the benefit of their patients.
Introduction
The article by Iverson and Gomez elsewhere in this issue appropriately emphasizes the prevalence of deep vein thrombosis (DVT) and the importance of its prevention and recognition in patients having plastic surgery. DVT is such a significant, but poorly appreciated, health care issue that the US Public Health Service underscored its importance in The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism , which it published in 2008. It is estimated that approximately 2 million cases of DVT occur in the United States annually, and that from 350,000 to 600,000 of those cases result in pulmonary embolus (PE), with 100,000 to 200,000 attributable deaths. An additional undetermined number of deaths may be attributed to nonhemorrhagic stroke in association with a nonfunctioning patent foramen ovale allowing passage of thrombus. The local signs and symptoms of DVT are caused by obstruction of venous outflow by thrombi, causing inflammation of the vein wall and inflammation and tenderness of the tissue surrounding the vein even before embolization into the pulmonary circulation. It is significant that approximately 50% or more of the DVT cases are silent, offering no early clinical signs or symptoms as warning. For those who survive the episode of DVT without those early clinical sign or symptoms, postthrombotic syndrome may also develop with significant long-term medical consequences.
From the numerous, well-documented reports in the literature, it is clear that DVT, silent or not, can be a significant problem in patients having plastic surgery. What is not so clear is what should be done about it. Several approaches to DVT prophylaxis are available and, despite suggested risk assessment algorithms, who should receive what treatment or combination of treatments has not been universally accepted across this specialty. The most recent and authoritative plastic surgery publication seeking to clarify this complex issue was the report from the American Society of Plastic Surgeons’ (ASPS) Venous Thromboembolism Task Force, Evidence-based Practices for Thromboembolism Prevention , which was approved by the ASPS Executive Committee for publication in July 2011. In conclusion, the report made the following recommendation:
Based upon the types of cases included in the literature review, Task Force members agreed that there was not enough evidence to make all-inclusive recommendations for plastic surgery prophylaxis medication, dosage, or length of prophylaxis. However, the task force agreed that some plastic surgery procedures warranted additional prophylaxis considerations; and accepted the premise that the surgical cases included in the orthopedic and general surgery literature search were similar enough in their anatomic location, degree of invasiveness and patient population to make them comparable (from a VTE risk perspective) to the following plastic surgery cases: major body contouring; abdominoplasty; major breast reconstruction; major lower extremity procedures; and major head/neck cancer procedure.” (VTE indicates venous thromboembolism.) Although there have been no randomized, controlled trials of chemoprophylaxis specific to plastic and reconstructive surgery, the validity of that approach was supported by the February 2012 publication of Antithrombotic Therapy and Prevention of Thrombosis, 9th edition: American College of Chest Physicians Evidence-based Clinical Practice Guidelines .
Although some clinical practice guidelines regarding risk stratification and prevention were recommended in that referenced report, no definitive standard of care was established. At this time, the most definitive reviews of the literature and most clinically applicable guidelines for DVT prophylaxis are found in publications from the ASPS and American Society for Aesthetic Plastic Surgery.
Regardless of the risk stratification or algorithm used, if chemoprophylaxis is selected as part of the regimen, a full understanding of the basics of thrombus formation, of the available agents and how and why they work, as well as their associated risks, benefits and alternatives, is critical to their safest and most effective use. That is the focus of this article.
Introduction
The article by Iverson and Gomez elsewhere in this issue appropriately emphasizes the prevalence of deep vein thrombosis (DVT) and the importance of its prevention and recognition in patients having plastic surgery. DVT is such a significant, but poorly appreciated, health care issue that the US Public Health Service underscored its importance in The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism , which it published in 2008. It is estimated that approximately 2 million cases of DVT occur in the United States annually, and that from 350,000 to 600,000 of those cases result in pulmonary embolus (PE), with 100,000 to 200,000 attributable deaths. An additional undetermined number of deaths may be attributed to nonhemorrhagic stroke in association with a nonfunctioning patent foramen ovale allowing passage of thrombus. The local signs and symptoms of DVT are caused by obstruction of venous outflow by thrombi, causing inflammation of the vein wall and inflammation and tenderness of the tissue surrounding the vein even before embolization into the pulmonary circulation. It is significant that approximately 50% or more of the DVT cases are silent, offering no early clinical signs or symptoms as warning. For those who survive the episode of DVT without those early clinical sign or symptoms, postthrombotic syndrome may also develop with significant long-term medical consequences.
From the numerous, well-documented reports in the literature, it is clear that DVT, silent or not, can be a significant problem in patients having plastic surgery. What is not so clear is what should be done about it. Several approaches to DVT prophylaxis are available and, despite suggested risk assessment algorithms, who should receive what treatment or combination of treatments has not been universally accepted across this specialty. The most recent and authoritative plastic surgery publication seeking to clarify this complex issue was the report from the American Society of Plastic Surgeons’ (ASPS) Venous Thromboembolism Task Force, Evidence-based Practices for Thromboembolism Prevention , which was approved by the ASPS Executive Committee for publication in July 2011. In conclusion, the report made the following recommendation:
Based upon the types of cases included in the literature review, Task Force members agreed that there was not enough evidence to make all-inclusive recommendations for plastic surgery prophylaxis medication, dosage, or length of prophylaxis. However, the task force agreed that some plastic surgery procedures warranted additional prophylaxis considerations; and accepted the premise that the surgical cases included in the orthopedic and general surgery literature search were similar enough in their anatomic location, degree of invasiveness and patient population to make them comparable (from a VTE risk perspective) to the following plastic surgery cases: major body contouring; abdominoplasty; major breast reconstruction; major lower extremity procedures; and major head/neck cancer procedure.” (VTE indicates venous thromboembolism.) Although there have been no randomized, controlled trials of chemoprophylaxis specific to plastic and reconstructive surgery, the validity of that approach was supported by the February 2012 publication of Antithrombotic Therapy and Prevention of Thrombosis, 9th edition: American College of Chest Physicians Evidence-based Clinical Practice Guidelines .
Although some clinical practice guidelines regarding risk stratification and prevention were recommended in that referenced report, no definitive standard of care was established. At this time, the most definitive reviews of the literature and most clinically applicable guidelines for DVT prophylaxis are found in publications from the ASPS and American Society for Aesthetic Plastic Surgery.
Regardless of the risk stratification or algorithm used, if chemoprophylaxis is selected as part of the regimen, a full understanding of the basics of thrombus formation, of the available agents and how and why they work, as well as their associated risks, benefits and alternatives, is critical to their safest and most effective use. That is the focus of this article.
Coagulation cascade
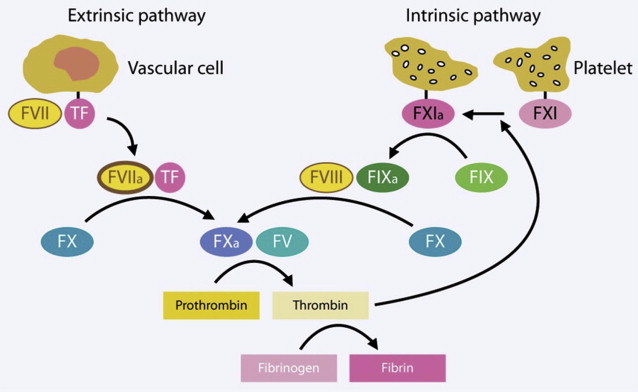
To understand the differences between the alternative chemoprophylaxis agents and how and why they work, at least a basic understanding of the coagulation cascade is necessary. The coagulation cascade is a series of enzymatic reactions that turns inactive precursors into active factors, the end result of which is the production of fibrin (factor I a [FI a ]), a protein that serves as a glue to bind platelets and other materials together in a stable clot. The cascade has 2 initially separate, but then converging, pathways: the extrinsic pathway (which initiates coagulation in response to tissue injury, such as trauma or surgery) and the intrinsic pathway (so named because all the elements required for blood clotting are present, even in the absence of injury).
These two pathways converge to become a common pathway with the activation of factor X (FX) ( Fig. 1 ). Active factors are indicated by the subscript a, whereas inactive precursors have no subscript.
Thrombus formation
The extrinsic pathway is initiated when tissue factor (TF), a transmembrane glycoprotein receptor in the subendothelial layers of blood vessels, is exposed to flowing blood following injury to the vessel. TF is rapidly bound by circulating factor VII (FVII). Binding is accompanied by autoactivation to FVII to form factor VII a (FVII a ). The complex of TF and FVII a converts factor X (FX) into its active form, factor X a (FX a ). FX a subsequently binds factor V (FV) to form prothrombinase, the enzyme responsible for converting prothrombin into thrombin. In turn, thrombin converts fibrinogen to fibrin, initiating the clot.
In the intrinsic pathway, factor XI (FXI), which is found on activated platelets, is autoactivated to factor XI a (FXI a ), which is capable of converting factor IX (FIX) to factor IX a (FIX a ). (Again, the subscript a denotes the active form of a factor.) In turn, FIX a activates FX in the presence of factor VIII (FVIII). At that point, the extrinsic and intrinsic pathways merge, with both causing the binding of FX a to FV to form prothrombinase, the subsequent conversion of prothrombin into thrombin, and the thrombin-mediated formation of fibrin from fibrinogen. The extrinsic pathway can feed back into the intrinsic pathway as a result of thrombin-mediated activation of FXI, thus providing additional clotting support to seal a vascular injury.
Intrinsic control of the coagulation cascade and all of its interactions is necessary to maintain hemostatic balance. The extrinsic control and manipulation of that cascade is the focus in chemoprophylaxis of DVT. However, there is a difference between arterial and venous thrombus formation. Arterial thrombi consist primarily of platelet aggregates held together by small amounts of fibrin. Because of that, strategies to inhibit arterial thrombogenesis focus primarily on drugs to disrupt platelet function. Venous thrombi are composed predominantly of fibrin and trapped red blood cells with few platelets. As a result, fibrinolytic agents are the focus for the treatment of venous thrombi. Although the development of new fibrinolytic agents has lagged behind that of antiplatelet and anticoagulant drugs, there are still many agents from which clinicians can currently choose for DVT chemoprophylaxis, and many others under development.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree