Fig. 30.1
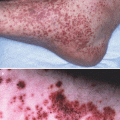
Lupus-specific skin disease. (a) Acute cutaneous LE. (b) Drug-induced subacute cutaneous LE secondary to rabeprazole (Aciphex) (personal unpublished observation, R. D. Sontheimer). (c) Classical discoid LE affecting the chin and lips. (d) Classical discoid LE affecting the scalp and external ears with scarring alopecia and postinflammatory hypopigmentation
Generalized ACLE is less common and presents as a widespread morbiliform eruption, often accentuated in a photodistributed pattern over the extensor aspects of the arms, forearms, and dorsal hands and fingers. Over the dorsal fingers during the early phase of the disease, the hair-bearing interphalyngeal areas are especially targeted while the knuckles are spared. Some patients experience an extreme form of ACLE that simulates toxic epidermal necrolysis (TEN), due to the intense lichenoid inflammation [15]. Of note this is one mechanism for the development of vesicular lesions in CLE.
LE patients can experience several types of vesiculobullous skin disease. In “bullous SLE,” patients typically with active SLE can present with vesicles (which appear similar to dermatitis herpetiformis) or bullae on the face, arms, and trunk [3]. Histopathological examination of the skin often reveals papillary dermal neutrophilic microabcesses as well as deposition of multiple immunoreactants at the dermal-epidermal junction. In some cases, these antibodies have been shown to bind type VII collagen, while in others the multiple immunoreactants are more consistent with non-specific immune complex deposition. Thus, these lesions clinically and histologically simulate dermatitis herpetiformis or epidermolysis bullosa acquisita, but the clinical distribution and multiplicity of immunoreactants and the presence of other features of LE help to distinguish these cases.
As “bullous SLE” does not share the histopathologic findings that are typical of CLE, it can be considered as a form of LE-nonspecific vesiculobullous skin disease. Vesiculobullous annular SCLE is an example of vesiculobullous LE-specific skin disease. Of note, vesiculation can also result from other blistering disorders, such as bullous pemphigoid, dermatitis herpetiformis, porphyria cutanea tarda, and pemphigus vulgaris that have rarely been reported to occur concordantly with LE [79–82].
Superficial ulceration of the oral or nasal mucosa can also occur in ACLE. These lesions are often asymptomatic, transient, and tend to occur on the hard palate (although virtually any area of the oral mucosa can be involved) [2, 16].
The lesions of ACLE are typically photosensitive and transient, usually lasting several days or weeks. Patients can concurrently develop SCLE, or, less commonly, DLE lesions. ACLE lesions do not scar but can result in predominate post-inflammatory pigmentary alteration, especially in dark-skinned patients.
The differential diagnosis for ACLE includes any dermatosis that can produce a red face, with common diagnoses being acne rosacea, dermatomyositis, seborrheic dermatitis, contact dermatitis, polymorphous light eruption (PMLE), and drug eruptions. Rosacea deserves special mention as a mimic of ACLE. In a study of patients referred to a dermatology clinic with a presumptive diagnosis of cutaneous LE, there were 21 that in fact had a different dermatological condition. Sixteen of these 21 patients had rosacea based on biopsy or diagnostic clinic features [2].
Subacute Cutaneous Lupus Erythematosus
First described as a distinct entity by Gilliam in 1977, SCLE is the prototype of a LE-specific skin disease that is defined by clinical, serological, and genetic features [10]. Clinically, these lesions present as either scaling papules or small plaques (“psoriasiform type”) (Fig. 30.1b) or scaling, annular and/or polycyclic plaques (“annular type”)—these forms are equally prevalent [2]. In general, one individual presents with one or the other type, though both forms can occur in a patient.
Lesions are characteristically photodistributed on the chest, back, extensor arms and V-area of the neck—it is the experience of the authors and others [92] that these lesions occur less commonly on the face compared to ACLE and DLE [2, 17]. Eighty-five percent of all SCLE patients report photosensitivity [18]. The inactive central portion of the radially spreading annular lesions is often hypopigmented. As in ACLE (see above), intense basovacuolar degeneration of epidermal keratinocytes can result in vesiculation and/or crusting, which usually occurs at the active edge of annular lesions. This can resemble TEN in its extreme form [19]. Lesions of SCLE typically heal without scarring, but permanent hypopigmentation and/or telangiectasias can occur. SCLE patients can also develop the lesions of ACLE or classic DLE. Localized facial ACLE has been reported to occur in 20 % of patients [3], while various reports document DLE lesions in 0–30 % of SCLE patients [11, 18]. In contrast to ACLE, SCLE lesions tend to be less transient, more scaly, less edematous, associated with more pigmentary change, and, as previously mentioned, less commonly affect the face. The absence of induration in SCLE lesions can serve as a clinical distinguisher from DLE and LE tumidus.
The differential diagnosis for SCLE includes psoriasis, dermatophyte infections, pityriasis rubra pilaris, polymorphous-light eruption (PMLE), nummular eczema, dermatomyositis, and mycosis fungoides. Annular lesions can be confused with granuloma annulare, erythema multiforme, and gyrate erythemas. However, the inactive centers of SCLE lesions are typically hypopigmented while those of other annular disorders are typically pigmented normally or hyperpigmentated.
Chronic Cutaneous Lupus Erythematosus
Classic DLE is the most common form of CCLE. These lesions begin as erythematous papules, which then develop scale and evolve into larger plaques covered by adherent scale that are usually associated with follicular plugging and peripheral hyperpigmentation. When the adherent scale is peeled back, follicle-sized keratotic spikes can be seen to project from the underside (the so-called “carpet tack sign”). The lesions expand slowly leaving central atrophy, scarring, telangiectasia, and depigmentation (Fig. 30.1c). Hyperpigmentation is often seen at the active borders of lesions. The combination of peripheral hyperpigmentation and central depigmentation is especially prominent in African American DLE patients. Some lesions of DLE can present only as macular hyperpigmentation, especially in Asian Indians [20].
DLE lesions occur most often on the face, ears (especially the conchae), scalp, V-area of the neck, and extensor aspect of the arms. Any facial structure can be involved, including eyebrows, eyelids, nose and lips. Periocular lesions are often misdiagnosed and can present as blepharitis, conjunctivitis, or periorbital edema [2]. Lesions can occur in the malar distribution, but their chronicity, epidermal change and scarring should distinguish them from the classic malar rash of ACLE. An acneiform pattern (often in the perioral area or the chin) that resolves with pitted scarring is rarely seen [2, 21]. Compared with ACLE or SCLE, DLE is less commonly reported to be associated with ultraviolet (UV) exposure. Patients are often unaware of the time lag (up to 4 weeks) following sun exposure, and many lesions do not occur in sun-exposed areas (e.g., hair-bearing scalp, conchal bowl of ears) [22, 23].
Scalp involvement occurs in 60 % of patients with DLE, with persistent activity resulting in permanent scarring (Fig. 30.1d). However, alopecia that is associated with DLE can be reversible when it is secondary to early inflammation of DLE; the telogen effluvium that represents an increase in underlying SLE activity; and alopecia areata that has been shown to be commonly associated with DLE [24].
DLE lesions are termed “localized” if they occur only on the head and neck, while lesions above and below the neck are referred to as “generalized”. Lesions can also occur on the palmar or plantar surfaces [25, 26], the nail unit [2, 27], in areas of trauma (the Koebner or isomorphic response) [28]. Follicular DLE lesions have been described, often around the elbow, and may be more common in African-American and Asian patients [28, 29].
Hypertrophic DLE is a rare variant in which hyperkeratotic lesions occur (often on the extensor extremities, upper back, and face). Histopathology can reveal features of squamous-cell carcinoma which can lead to confusion regarding diagnosis [115]. Even if patients have classic DLE lesions elsewhere, the clinician should still be aware that squamous-cell carcinoma can develop in long-standing, scarring DLE lesions [30, 31].
Well-developed lesions of DLE do not usually present a problem with differential diagnosis, although early lesions can be confused with PMLE, granuloma faciale, sarcoidosis, cutaneous lymphoid hyperplasia, lupus vulgaris, angiolymphoid hyperplasia with eosinophilia, and tertiary syphilis.
Mucosal DLE occurs in approximately 25 % of CCLE patients [16]. Oral lesions tend to occur on the buccal mucosa, and less commonly on the palate, gums, and tongue. Lesions have a sharply marginated, scalloped white border with central erythema. Central areas can erode, although lesions are typically painless. The surfaces of well-developed plaques on the palate can have a meshwork of raised hyperkeratotic strands giving a “honeycomb” appearance [16]. Fixed mucosal DLE lesions can be distinguished clinically from the transient superficial mucosal ulcerations that are often seen in active SLE patients. The lips can be involved with well-defined plaques or a diffuse cheilitis [3]. Such lesions can degenerate into squamous cell carcinoma [32]. Involvement of the nasal, conjunctival, and genital mucosa can occur [33].
Chilblain LE is characterized by red-purple patches or papules on the toes, fingers, and/or face that are precipitated by cold or damp climates. At the onset these lesions are clinically indistinguishable from simple chilblains (or, pernio) lesions that occur in healthy individuals [34, 35]. However, chilblains LE lesions tend to evolve into more classic acral DLE lesions, and it is postulated that this may be the result of a Koebner phenomenon in otherwise typical lesions of perniosis [3]. Differential diagnosis includes other cold-induced vasculopathies, such as cold agglutinin disease or cryoglobulinemia.
LE profundus (syn. LE panniculitis) is a form of CCLE characterized by inflammation in the lower dermis or subcutis. This lesion occurs more commonly in women and is seen in 1–3 % of SLE patients [3]. Approximately 70 % of patients will have overlying DLE lesions [36, 37]. Some have used the term “LE profundus” to specify those lesions that have concurrent overlying DLE activity and the term “LE panniculitis” to refer to lesions displaying only subcutaneous inflammation. However, this is not a universally accepted convention.
LE profundus/panniculitis lesions are characterized by firm, deep nodules with initially normal appearing overlying skin. With time, the nodules resolve and draw the surface of the skin inward, leaving deep, saucerized depressions. Lesions tend to occur in the head, upper arms, buttocks and thighs. Rarely, this entity can present as periorbital edema. Dystrophic calcification can occur in older lesions. Breast lesions (“lupus mastitis”) can be confused with carcinoma [2]. Persistent, extensive LE profundus/panniculitis lesions of the breast can necessitate mastectomy. Early lesions can be confused with morphea, while other forms of panniculitis (subcutaneous panniculitic T cell lymphoma, sarcoidosis. factitial or traumatic panniculitis, subcutaneous granuloma annulare) and lipoatrophy (partial lipodystrophy associated with autoimmune disease, drug-induced lipoatrophy, HIV-associated lipoatrophy) must be ruled out.
Typical lesions of lupus erythematosus tumidus (LET) are succulent, edematous papules and plaques that arise due to accumulation of dermal mucin. These lesions are found with decreasing frequency on the face, back, arms, and chest [38, 39]. In some individuals, large annular edematous plaques can be seen. The largest series reported resolution with no or mild topical treatment in nearly half of cases [39], although it has since been questioned that this series may have included many patients with PMLE [38]. It is possible that this might account for the extreme photosensitivity that was reported in these patients [39]. Other authors report these lesions to be chronic and difficult to treat and such patients are typically ANA-negative. Most affected patients do not have SLE [38–41].
Lesions are characterized by perivascular and peri-appendageal lymphocytic inflammation with dermal mucin deposition. Unlike other forms of LE-specific skin disease, there is absence of basal vacuolar changes in 80–100 % of cases, with positive cases showing only focal and sparse keratinocyte necrosis [38, 42]. This fact has led to discussions about how to best categorize LET. Some authors have characterized LET as a separate category of CLE called “intermittent CLE” due to histological differences and lack of scarring potential seen in other forms of CCLE [3]. LET lesions must be differentiated from PMLE, Jessner’s lymphocytic infiltrate, atypical lymphoid infiltrates, myocosis fungoides, reticular erythematous mucinosis, DLE, SCLE, and figurate erythemas. Some have argued that LE tumidus and Jessner’s lymphocytic infiltrate in reality cannot be clearly be distinguished [39].
Laboratory Abnormalities
Little data are available concerning laboratory assessment of patients with ACLE. It is assumed that this would closely parallel the data that are available for patients with SLE.
Approximately 60–80 % of SCLE patients have detectable antinuclear antibodies (ANAs) with a speckled/particulate ANA pattern being most common [43]. This disease is characterized by positive anti-Ro/SSA antibodies, present in 40–100 % of patients, depending on the assays used [44, 45]. Anticardiolipin antibodies are present in 10–16 % [46]. Rheumatoid factor is present in one third of SCLE patients [44], and some patients initially present with rheumatoid arthritis long before a diagnosis of SCLE is made. Sm, dsDNA, and U1RNP antibodies are present in 10 % of SCLE patients [44]. Anti-thyroid antibodies were reported in 18 % [47] and 44 % [48] of SCLE patients. Depending on the presence of SLE disease activity, cytopenias, hypergammaglobulinemia, proteinuria, hematuria, and depressed complement levels can also be seen.
In DLE, low ANA titers/levels (e.g., ≤1:40) are present 30–40 % of the time in assays that employ human tumor cells as substrates. However, higher titers that are typically seen in SLE (≥1:160) are rarely encountered in patients having isolated forms of DLE [2]. Anti-Ro/SSA antibodies are occasionally found, but the presence of anti-Sm, dsDNA, and La/SS-B antibodies is uncommon [49]. Fewer than 10 % of patients have IgG anticardiolipin antibodies [50]. A small percentage of DLE patients will have positive rheumatoid factor, slight depression in complement, and leucopenia (see below). Antinuclear antibodies are present in approximately 75 % of patients with lupus profundus/panniculitis [2]. The frequency of ANAs in patients with chilblains lupus was reported as 9 out of 14 patients, with anti-dsDNA antibodies in 4 of 14 patients—these numbers might be an overestimate [35]. Anti-Ro/SS-A antibodies have been variably found in these patients, and some authors have suggested that this is a marker for this disorder [35, 51].
Relationship to Systemic Disease
For ACLE, as it is generally presumed that this is a fundamental component of SLE, there are not many data regarding the relationship of ACLE and SLE disease activity. One study suggests that the course of rash severity parallels SLE activity [12]. Interestingly, authors have failed to support an association with renal or CNS disease, although this has not been studied adequately [2]. One small study indicates that SCLE and DLE patients with normal lymphocyte counts are unlikely to have SLE [52].
Approximately 50 % of patients with SCLE meet American College of Rheumatology (ACR) criteria for SLE [44, 53]. However, severe systemic disease (i.e., nephritis, CNS disease) develops in only 10 % of SCLE patients [44]. Some data support that the papulosquamous form of SCLE is more associated with renal involvement [2]. As stated above, rheumatoid arthritis has been reported to precede as well as follow a diagnosis of SCLE. In addition, 3–12 % of SCLE patients will later develop Sjögren’s syndrome [2]. Finally, there are some reports that suggest that SCLE might be a paraneoplastic syndrome [2]—due to the paucity of cases, a causal relationship has not been proven.
Historically it has been felt that 5–10 % of patients presenting only with DLE lesions will eventually develop SLE [2]. Two more recent prospective studies suggest the risk may be in the 10–20 % range [1, 4] However, a minority of those that eventually develop SLE by criteria experience moderate or severe disease [4]. Risk factors for progression include lesions above and below the neck, unexplained anemia, leucopenia, persistently positive high-titer ANA, hypergammaglobulinemia, and positive lupus band test of nonlesional skin [54]. Patients with evidence of nephropathy or arthralgias are also at increased risk of having SLE [55]. Similar to SCLE, patients with lupus panniculitis have a 50 % chance of having SLE, although this is usually mild with only 10 % meeting strict ACR criteria for SLE [2].
In patients with known SLE, the presence of CCLE lesions (namely DLE, lupus panniculitis, chronic mucosal plaques), appears to be associated with less severe systemic disease [2]. A more recent study suggested SLE patients with DLE had a decreased risk of arthritis and pleuritis but no effect on risk of nephritis [5].
Histopathology
The histopathology of CLE will be mentioned only briefly as it has been described in detail elsewhere [2]. In general, ACLE, SCLE and DLE have similar features that do not allow for distinction between the subsets of CLE. Characteristic findings include liquefactive degeneration of the epidermal basal-cell layer, variable hyperkeratosis, dermal edema and mucin deposition, and mononuclear cell infiltration around the dermal-epidermal junction and dermis. This infiltrate consists mainly of CD3+ (both CD4+ and CD8+) cells, with other cell types including histiocytes and plasmacytoid dendritic cells (see below). In DLE, the dermal infiltrate is generally denser and can extend more deeply into the reticular dermis. In addition, DLE lesions can demonstrate follicular plugging and more pronounced basement membrane thickening.
Variable deposition of immunoglobulin (IgM, IgG, IgA in decreasing frequency) and complement components can also be detected at the basement membrane zone of lesional skin. The frequency and intensity with which this is detected varies between studies, anatomic location of skin biopsies, and type of CLE [2].
Hypertrophic forms of DLE are characterized by a greater degree of epidermal acanthosis and hyperkeratosis. Notably, some areas can have features of squamous cell carcinoma or keratoacanthoma. Lupus pannicultis/profundus generally spares the dermal-epidermal junction (if overlying DLE is not present), and is characterized by a lobular panniculitis and perivascular mononuclear cell infiltrate [2]. The infiltrate in the fat is composed of histiocytes and lymphocytes (sometimes forming nodules) and can show variable hyaline-fat necrosis or calcification. LE tumidus shows a perivascular and periadnexal lymphocytic infiltrate with dermal mucin deposition. Studies show focal spotty keratinocyte necrosis in 0–20 % of cases [38, 42]. Chilblain LE shows basal vacuolar degeneration, superficial dermal edema, and a perivascular lymphocytic infiltrate. Some authors conclude that these entities can be distinguished by histopathology, with idiopathic chilblains being characterized by perieccrine inflammation and spongiosis [56, 57].
Pathogenesis
Most of the work pertaining to pathogenesis of cutaneous lupus relates to those forms of CLE that are characterized by interface dermatitis (i.e., ACLE, SCLE, DLE). Thus, this section will focus on these manifestations of CLE. In addition, much of the discussion will not distinguish between the different types of CLE, except for when specifically noted in the text.
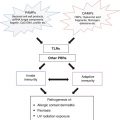
Before considering the etiology of LE-specific skin disease, it is interesting to consider its relationship with disease mechanisms that are associated with SLE. Evidence for a pathogenic relationship between cutaneous and systemic disease includes the association of LE-specific skin disease with SLE as well as the fact that, even in “skin-limited” disease, certain characteristic T and B lymphocyte abnormalities can be found systemically that mirror those seen in SLE [58, 59]. The general concept that genetic susceptibility (i.e., HLA haplotypes) and environmental triggers (infection, medication, ultraviolet light) result in a loss of immunologic self-tolerance which then is manifested by generation of autoantibodies and antigen-specific T lymphocytes that mediate tissue injury is likely operative in both cutaneous and systemic disease. However, it should be noted that there is no definitive evidence at present that demonstrates the cutaneous inflammation of CLE is due to an autoimmune response to antigen(s) in the skin. However, studies showing an oligoclonal expansion of T cells in the CCLE lesions are suggestive of an antigen driven reaction either in the skin or periphery [60, 61]. Interestingly, there is no evidence for this in infiltrates of lupus panniculitis [62].
Various genetic abnormalities are associated with different forms of LE-specific skin disease. Several HLA haplotypes have been associated with ACLE, SCLE, and DLE [3]. This implicates a role of T lymphocytes, and may relate specifically to their role in providing help for antigen-specific B cell responses, as a particular HLA B8 DR2 (DRB1*1501) DR3 (DRB1*0301) extended haplotype correlates with the anti-Ro/SS-A response [2]. A polymorphic variant in the TNF-α promoter that is associated with increased TNF-α production is highly associated with SCLE and neonatal LE [63, 64]. Genetic deficiencies in complement components, such as C2, C3, C4 and C5, have been associated with SCLE and/or DLE [2]. The role of C1q seems important, as complete congenital genetic deficiencies of this protein are a strong risk factor for photosensitive SLE [2]. In addition, a polymorphism in the C1QA gene is associated with SCLE [65].
Environmental factors play a role in the pathogenesis of CLE. The paramount role of UV irradiation is discussed below. Assuming that ACLE is triggered by the same mechanisms as for SLE, chemicals such as L-canavanine present in alfalfa sprouts (which induce SLE) may be important [2]. Infections, especially those caused by viruses, are also triggers for SLE. Multiple medications have been associated with the clinical induction of SCLE and less so with DLE [2, 116, 117].
It has been proposed that these may do so via inducing photosensitivity, which might result in disease activity via UV-specific mechanisms or simply via the Köebner phenomenon that results from photodamage. Although numerous drugs can induce SLE (e.g., procainamide, hydralazine, isoniazid), drug-induced SLE is typically not associated with cutaneous findings. Similarly, trauma appears to induce a Köebner phenomenon, especially in DLE patients. Smoking has been implicated as a risk factor for the development of SCLE and DLE [2]. It is unclear if this reflects a primary role for smoking in the disease process or simply results from its known association with antimalarial resistance.
Any consideration of the molecular pathogenesis of CLE must involve consideration of the role of ultraviolet (UV) light. Evidence for the role of UV light in CLE is strong: first, most CLE lesions are in photo-exposed regions of the body; second, 50 % of patients with lupus report photosensitivity; third, 54 % of patients with CLE demonstrate UV photoprovocation of their lesions in the lab [66]. Finally, an immune response against UV-altered DNA has been shown to occur in both mouse models of lupus and patients with SLE. However, the relative importance of UV light in the pathogenesis of likely multiple genetic and phenotypic forms of CLE is not currently known. It is likely that other environmental triggers (i.e., infection, cellular injury, medications) can lead to CLE as well, although the mechanisms for these are not well worked out. Thus, although most of the data presented related to how UV light induces CLE, other forms of keratinocyte damage and/or activation of the cutaneous immune system can be applied in the final model.
Although it is clear that UV light (UVA or UVB) can induce CLE lesions in susceptible patients, the mechanistic link between UV exposure and the cutaneous inflammation that is observed is still not clear—many of the proposed mechanisms may be operating simultaneously. Ultraviolet light can generate neoantigens, such as UV-modified DNA; when injected into mice, this altered DNA can cause lupus like disease [66]. Another mechanism might be the ability of UV light to induce apoptosis of keratinocytes by multiple mechanisms, including oxidative damage to mitochondrial membranes, damage to DNA, induction of p53, activation of membrane death receptors (Fas), and sensitization to TNF-α (and TNF-related apoptosis inducing ligand, TRAIL). Normally, the immunologic clearance of apoptotic cells is a non-inflammatory event. An increased rate of formation and/or decreased ability to “clear” these apoptotic cells, however, can lead to early necrosis of cells and result in their capacity to stimulate the immune system. This occurs via multiple mechanisms, including the ability of necrotic elements to induce maturation and activation of local antigen presenting cells [67]. Necrotic cells can release pro-inflammatory mediators such as high mobility group 1 (HMG1) protein, which is found in high levels in the skin of CLE patients [68]. The C1QA and other complement deficiencies that are associated with CLE suggest that these patients might have a defect in removal of apoptotic cells. Is there evidence of defective clearance of apoptotic keratinocytes in CLE? Data are conflicting in this regard [69, 70]. Whether or not CLE patients have an increased number of apoptotic cells, it is still possible that these cells could somehow lead to inflammatory sequelae in CLE patients. Indeed, a recent observation suggests that detectable inflammation correlates with the presence of apoptotic cells in the near vicinity in CLE patients [70].
If one accepts that in CLE patients, UV-damages keratinocytes with an inflammatory response, then how might such a response be propagated? One theory is that the binding of circulating autoantibodies against cellular constituents of dying keratinocytes results in a local inflammatory response (via FcR-dependent or complement-dependent mechanisms) [66]. Since the seminal observation by Casciola-Rosen and colleagues that UV light induces translocation of intracellular keratinocyte antigens to the cell surface (in structures called blebs) [71], and that these antigens include the SSA/Ro and SSB/La antigens that are the targets of commonly found autoantibodies in CLE (especially SCLE), the ability to definitively link this process with the clinical findings of CLE has eluded investigators. In fact, the frequency and/or titers of SSA/Ro antibodies do not always correlate with skin activity in CLE patients [66]. However, it has recently been suggested that SSA/Ro autoantibodies are capable of interfering with the clearance of apoptotic cells [119]. Still, it might be that the critical antibodies are not being measured, and that apoptotic-modified forms of these antigens are the critical targets [66]. In general, LE patients have antibodies that appear to be directed against antigens involved in the cellular-stress response and heat shock response. Whatever the answer, it seems unlikely that such autoantibodies play a role in initiating CLE disease, as the deposition of antibodies in CLE tends to follow, not precede, the cellular inflammation [72].
UV light can also promote inflammation by inducing the secretion of cytokines and chemokines, as well as upregulating the expression of adhesion molecules [73]. UVB induces IL1-α and TNF-α in the epidermis. These cytokines induce release of IL-6, PGE2, IL-8 and granulocyte-monocyte colony-stimulating factor (GM-CSF) by keratinocytes [66]. The end result is activation of Langerhans cells, chemotaxis of lymphocytes, and up-regulation of adhesion molecules on keratinocytes (ICAM-1) and endothelial cells (ICAM-1, VCAM-1, E-selectin). UVB irradiation also induces expression of chemokines, such as CCL5, CCL27 and CXCL8 [74]; these have all been found at high levels in CLE skin, and function to recruit memory T cells into the vicinity. Via production of oxygen-free radicals, UVA upregulates ICAM-1 in keratinocytes, but, because it can penetrate deeper into the skin, is also able to upregulate vascular endothelial ICAM-1 and E selectin, which allows leukocyte extravasation into the skin [66]. In addition, UVA is able to induce secretion of IL-12, a potent immunostimulant [66].
T lymphocytes are likely to play a major pathological role in CLE. Skin infiltrates consist primarily of CD3+ T cells, both CD4+ and CD8+ [66]. CD4+ cells appear early in the skin, with CD8+ appearing later. Recent data suggest that skin homing (CLA+) CD8+ cytotoxic cells might be responsible for the scarring that is seen in CCLE [75]. These cells are seen predominantly in the skin of DLE patients (as opposed to other CLE subsets), and an expanded population of circulating, CCR4+, CLA+ CD8+ cells is associated exclusively with generalized DLE [76]. These cells secrete granzyme B, a serine protease that causes tissue death and could conceivably account for injury (and resulting scar) to adnexal and epidermal structures. IFN − α may also play a role in this process, as local IFN-α activity was found to be correlated with CD8+ cell infiltration. Further evidence for the role of activated T cells in CLE comes from the increased expression of HLA-DR and CD25 (both activation markers) on circulating CD4+ and CD8+ cells in patients with DLE and SCLE—furthermore, these levels correlated with cutaneous disease activity [58, 59].
The cytokine expression pattern found in DLE lesions is representative of a mixed Th1 and Th2 profile. Lesions are characterized by high levels of IL-1, IL-2, IFN-γ, TNF-α, IFN-α, IL-5 and IL-10 [66, 67]. TNF-α is found in increased levels in the skin of DLE and SCLE patients and serum levels correlate with disease [77, 78]. TNF-α can promote many of the findings seen in CLE: translocation of SSA/Ro to the cell surface of keratinocytes; apoptosis of keratinocytes; hyperkeratosis; and increased expression of adhesion molecules that favors cutaneous leukocyte infiltration [79]. In addition to the effects mentioned above, IFN-γ has been shown to cause keratinocyte apoptosis [80]; a mouse strain that overexpresses IFN- γ in keratinocytes results in clinical features of SLE and cutaneous inflammation [66]. IL-1 is generated by keratinocytes in response to UV light, and transgenic expression of IL-1 in mice results in hair loss, scaling, and focal inflammatory lesions [66]. Keratinocytes also express an antagonist of the IL-1 receptor, and null alleles of this protein have been reported in SLE patients with photosensitivity as well as in CCLE patients [66].
The role of the innate immune system in CLE is beginning to be explored. It has recently been discovered that IFN-α plays an important role in the pathogenesis of SLE [81]—evidence shows that the source of this IFN-α is the plasmacytoid dendritic cell (pDC). High numbers of pDC have been detected in the lesions of CLE [82], with accompanying high levels of local IFN-α activity [76, 82–84]. IFN-α is known to induce the chemokines CXCL9, CXCL10, and CXCL11, which are also at high levels in CLE skin [74]. The ligand for these chemokines, CXCR3, is found on infiltrating T cells in CLE. Thus, local emigration of activated pDC to the skin of CLE patients might represent the mechanism whereby T cells initially migrate into cutaneous lupus lesions. At present it is unclear what the signals are that cause pDC migration to the skin; however, it is tempting to speculate that locally deposited immune complexes (i.e., the lupus band) might play a role in their ongoing local activation, since it is known that immune complexes containing nucleic acid (from apoptotic cells) activate pDC via toll-like receptors (TLRs), resulting in the production of IFN-α [85].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree