CHAPTER 61 Cross-Linked Polyethylene
Highly cross-linked and thermally treated ultra–high-molecular-weight polyethylene (UHMWPE) (hereafter, cross-linked polyethylene) has been used in total hip arthroplasty in the United States since the late 1990s. Since that time, cross-linked polyethylene has steadily replaced conventional polyethylene for hip arthroplasty.1 According to recent surveys, approximately 70% of hip replacements performed in the United States employ a cross-linked polyethylene liner.2 For 2006, approximately 250,000 primary and revision hip total procedures were projected to be performed annually in the United States.3 Therefore an estimated 175,000 total hip arthroplasty patients in the United States are now considered to benefit from cross-linked polyethylene technology every year. Considering the historical growth in the use of cross-linked polyethylene since its clinical introduction in 1998,2 combined with the projected growth in hip arthroplasty,3 over one million patients will have received a cross-linked liner in the United States by 2007.
Cross-linked polyethylene has come to be widely accepted by surgeons as an alternative to hard-on-hard bearings for improving the wear resistance of hip arthroplasty.4 As we shall see subsequently in this chapter, the scientific foundation for such enthusiasm for cross-linked polyethylene warrants critical appraisal. In the 1990s, polyethylene wear, particle-mediated osteolysis, and aseptic loosening were regarded as among the most important clinical problems limiting the longevity of hip replacements.5,6 Dumbleton and colleagues7 demonstrated from review of the literature that osteolysis was likely in patients with head penetration into the bearing of more than 0.1 mm/year and unlikely in patients with head penetration of less than 0.05 mm/year. Therefore alternative bearings were urgently needed to reduce wear and secondary osteolysis, thereby improving long-term survivorship of the replacement.
During the mid-1990s, retrieval analyses also identified oxidation in the polymer, attributed to the gamma sterilization process together with long-term shelf storage or oxidation in vivo.8–11 The association between oxidation and clinical performance of hip arthroplasty was not completely clear.12 However, it was considered desirable in certain scientific circles to engineer polyethylene materials for not only wear resistance, but oxidation resistance as well.13,14 It is important to keep in mind that what we refer to as “cross-linked polyethylenes” throughout this chapter are actually fabricated in two steps: the first cross-linking step is designed to improve wear resistance, whereas the second thermal processing step is designed to achieve a combination of oxidation resistance and mechanical properties.
Because both cross-linking and thermal processing can deleteriously influence mechanical properties, such as fracture resistance, creep, and ultimate strength, the precise details employed in these manufacturing steps have been the subject of considerable scientific debate and controversy over the past 8 years. The polymer science and technology used to produce cross-linked polyethylene have been summarized in a monograph.15 Therefore in the current chapter we will provide the practicing surgeon or resident with an overview of the current concepts used to design cross-linked polyethylenes for hip arthroplasty. Readers interested in a more detailed treatment of cross-linked polyethylene technology are referred to the UHMWPE Handbook15 or a frequently updated online resource dedicated to this subject (www.uhmwpe.org).
Because of its improved wear-resistance, cross-linked polyethylene is now regarded as a desirable technology for hip articulations. The same cannot be said unequivocally for the use of highly cross-linked polyethylene in total knee replacement, where concerns about reduced material properties have continued to limit its clinical acceptance.16 In this chapter, we focus specifically on applications of cross-linked polyethylene in the hip. We begin with an overview of the basic science concepts and terminology surrounding cross-linked polyethylene and the two main thermal processing techniques, which involve either annealing or remelting the polymer after cross-linking. The second part of the chapter is a critical assessment of the peer-reviewed literature on the subject of femoral head penetration and wear in cross-linked polyethylenes measured in clinical studies.
BASIC SCIENCE
Chemical Structure and Molecular Weight
There has been some confusion in the historical arthroplasty literature, much of it written by Charnley,17 in which what is now considered UHMWPE was historically referred to as high-density polyethylene (HDPE) or polythene. Today, high-density polyethylene refers to a material with a molecular weight of 100 to 250,000 daltons and is suitable for milk jugs, not artificial joints. In a hip simulator, HDPE has a wear rate that is four times higher than that of UHMWPE.18 It is clear, therefore, that what we consider today to be modern HDPE has never been used clinically.
We will not dwell more on the nomenclature or history of UHMWPE throughout its four decades of uninterrupted clinical use, as these topics are treated in a previous monograph15 and are also discussed online. For the purposes of this chapter, we are concerned primarily with modern UHMWPE, which for convenience we will continue to refer to simply as polyethylene.
Crystallinity
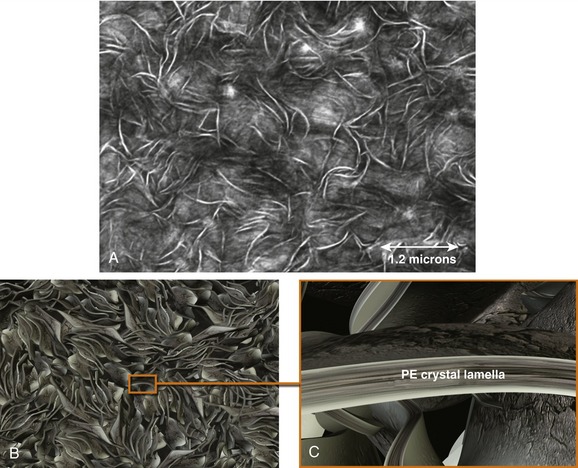
Crystallinity is an important attribute of all polyethylenes, including cross-linked polyethylene. The molecular chains in polyethylene have a natural tendency (driven by thermodynamics) to preferentially fold up against themselves whenever possible, hindered by the considerable crowding and thermal jostling presented by adjacent molecules. Regions of the polymer with folded chains are referred to as crystallites. Individual crystallites in polyethylene are microscopic and can typically be visualized only after staining using an electron microscope, as shown in Figure 61-1. To the naked eye, crystalline regions diffract visible light, giving polyethylene its white appearance. In the transmission electron microscope (see Fig. 61-1A), the crystalline regions appear like thick white lines, whereas the regions with randomly oriented polymer chains, referred to as the amorphous regions, appear dark gray.
In polyethylene, the crystallites have a particular “lamella” shape. If we were to dissolve away the amorphous regions, the crystalline lamellae in polyethylene would look something like twisted, interconnected sheets, as shown conceptually in Figure 61-1B. A close-up illustration of a crystal lamella is shown in Figure 61-1C. The molecular chains are oriented perpendicular to the plane of the lamella and may emerge to connect with adjacent lamellae. These connective polymer chains (not shown) are referred to as tie molecules. In particular, it is thought that tie molecules, or entanglements, contribute greatly to the inherent wear resistance of polyethylene.
Cross-Linking
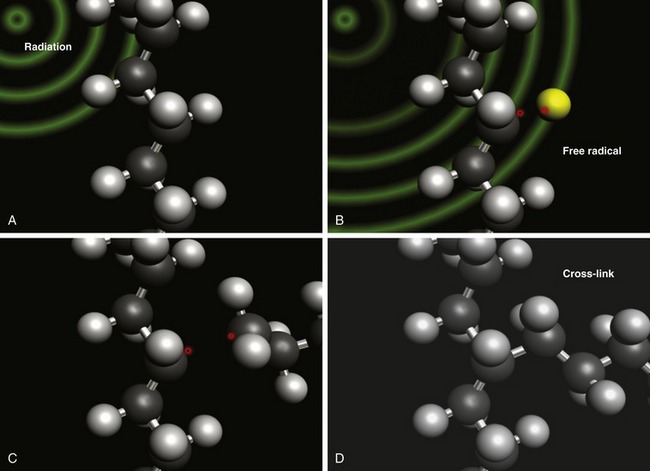
The mechanism of radiation cross-linking is schematically illustrated in Figure 61-2. The first step involves irradiation of the polyethylene molecule (see Fig. 61-2A). Next, irradiation produces a hydrogen radical, leaving a so-called “free” radical on the polyethylene molecule (see Fig. 61-2B). Actually, the radical on the polymer chain has extremely limited mobility and is hindered by the adjacent molecule. It may be more properly referred to as a “macroradical.” For cross-linking to occur, macroradicals must be present on adjacent polyethylene molecules, and the molecules must be mobile (see Fig. 61-2C). When the adjacent radicals react, a covalent bond, or cross-link, is formed between the two polyethylene molecules (see Fig. 61-2D).
The extent of cross-linking in polyethylene is proportional to the absorbed dose of radiation. Historically, polyethylene bearings were gamma sterilized at a dose of 25 to 40 kGy. This dose resulted in the formation of some cross-links. Saturation of cross-linking was achieved only at approximately 100 kGy of absorbed dose. Today, cross-linked polyethylenes are processed with a total dose ranging from 50 to 105 kGy, depending on the manufacturer.15 In general, increasing the dose provides a proportional improvement in wear resistance, as quantified by a hip simulator, with diminished benefits observed above 100 kGy.19,20 The precise reasons why cross-linking improves wear resistance are still not completely understood but are thought to derive mostly from the improved resistance to uniaxial orientation that accompanies cross-linking.21,22 The dominant wear mechanism for polyethylene in hips involves preferential orientation, followed by debris formation resulting from cross-shear.23 Because cross-linking makes it more difficult for the material to become oriented preferentially, the usual wear mechanism in hip replacements is disrupted.
One choice an implant designer has to make is the method of cross-linking (e.g., gamma versus electron beam radiation). If irradiation is to be carried out using electron beam irradiation, the designer must consider the additional factor of irradiation temperature, because the rate of energy dissipation increases the temperature above the melting temperature.20 Of the five major orthopedic manufacturers currently producing cross-linked polyethylene implants, one has chosen electron beam irradiation, whereas the other four use gamma radiation cross-linking.
Although resistance to multiaxial deformation is desirable for wear resistance in a hip application, cross-linking reduces the ductility and resistance of polyethylene to uniaxial tension. The resistance of polyethylene to fatigue and fracture also decreases with increased dose.24–26 Therefore the dose of a cross-linked material is chosen with not only wear resistance in mind, but also the impact the dose will have on other desirable mechanical properties, such as ductility and fracture resistance. Because cross-linking improves certain properties at the expense of others, developers of orthopedic implants must balance cross-linking with the desire to maintain mechanical properties and/or oxidation resistance.