Fig. 14.1
MRI of the right leg in primary lymphedema. Axial T2-W fat-saturated (a) and T1-W fat-saturated, contrast-enhanced (b) MR images of the calf demonstrate extensive circumferential subcutaneous soft tissue thickening and reticular (honeycomb) pattern above the fascia with thickening of the subcutis and dermis. Note reticular post-contrast enhancement suggestive of lymphangitis/cellulitis. Coronal T1-W (c) and T2-W fat-saturated (d) MR images of right calf demonstrate predominantly subcutaneous extrafascial distribution of fluid and fat accumulation; a classic feature of chronic primary lymphedema
MRI can also be useful in the differential diagnosis of lymphedema [8–10]. In edema due to venous disease both the epifascial and subfascial compartments may be affected; the characteristic reticular pattern may or may not present. While in lipidemia, the fat accumulation occurs without signs of lymphatic congestion or reticular appearance [10]. The anatomic details provided by MRI may complement the functional assessment provided by lymphoscintigraphy and can be occasionally necessary to establish the diagnosis [11].
MRI may be helpful in differentiating the various causes of lymphatic obstruction in secondary lymphedema by demonstrating dilated lymphatic trunks and identification of abnormal lymph nodes. Malignant nodal involvement can be assessed further by lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI). These nanoparticles produce a significant susceptibility effect which can be detected as a drop in signal intensity on T2-weighted images [12]. Within a normal lymph node, nanoparticles accumulate within the reticuloendothelial system (phagocytized by macrophages) and show homogeneous uptake resulting in dark signal on T2-weighted images. In a node which is either partially or completely infiltrated by malignant cells, there is absence of functioning macrophages, leading to a lack of nanoparticle uptake resulting in focal or complete area of bright signal intensity on T2-weighted images. This technique is highly effective in identifying metastases in non-enlarged and partially replaced nodes; however, due to the negative-contrast nature of the detection, small lesions can be missed [13]. In one study, sensitivity and specificity of LNMRI was 76.5 % and 98.4 % in diagnosing nodal metastasis [14]. Lymphatic flow velocities can be assessed in lymphedema by visualization of lymphatic flow using principles of spin labeling MR imaging, and thus, lymphedema etiogenesis and therapies may be interrogated without exogenous contrast agents [15].
Magnetic Resonance Lymphography (MRL)
Magnetic resonance lymphangiography (MRL) is a recently added technique in which gadolinium-based, MRI contrast agent is injected for the visualization of lymphatic vessels in patients with primary and secondary lymphedema [16, 17]. A mixture of MRI contrast agent (gadobenate dimeglumine 0.1 mmol per kilogram or gadopentetate dimeglumine 0.2 mmol per kilogram of body weight) and 2 mL of Bupivacaine hydrochloride 0.25 % or Mepivacaine hydrochloride 1 % is injected intracutaneously into the interdigital webs of the dorsal aspect of both feet. Before MR lymphangiography, the extent and distribution of the lymphedema is evaluated using a heavily T2-weighted 3D turbo spin-echo sequence. For MR lymphangiography a 3D spoiled gradient-echo sequence [volumetric interpolated breath hold examination (VIBE)] is used. The three stations are first imaged without contrast material and subsequently repeated at 5, 15, 25, 35, 45, and 55 min after intracutaneous application of contrast. To emphasize the gadolinium-containing structures, baseline images are subtracted before 3D maximum intensity projection (MIP) reconstructions are calculated.
In one study, contrast MRL was capable of evaluating the anatomical and functional status of lymphatic vessels and lymph nodes in primary and secondary lymphedema by real-time visualization of enhanced lymph flow in lymphatic channels and within lymph nodes. In primary lymphedema, there were three major types of lymphatic system malformation: (a) only lymph nodes affected, (b) only lymph vessels affected, and (c) both lymph vessels and lymph nodes affected. In secondary lymphedema, MRL demonstrated tortuous and dilated collecting lymphatics in lymphedematous limbs [18].
In other study, diagnostic accuracy of magnetic resonance imaging (MR-lymphangiography) was calculated relative to the lymphoscintigraphy gold standard for assessment of focal lesions of the peripheral lymphatic system. MR-lymphangiography had sensitivity of 68 %, specificity of 91 %, positive predictive value of 82 %, and negative predictive value of 83 %. There was substantial correlation of results between the two modalities [19].
MR lymphangiography using interstitial injection of gadofosveset trisodium (Ablavar®, Lantheus Medical, North Billerica, MA) alone or premixed with 10 % human serum albumin (HSA) was used to visualize thoracic duct (TD) in a pig model [20]. Intradermal injection of nano-sized gadolinium-labeled dendrimer was also shown to rapidly opacify the deep lymphatic system, including the thoracic duct, in mice and pigs [21].
Computed Tomography
Although MRI is the preferred modality for assessing lymphedema, computed tomography (CT) can also be used, particularly when MRI cannot be technically or safely performed (e.g., uncooperative patients, unstable cardiovascular or respiratory status, contraindications to MRI). Acquiring CT scan studies is faster and can be performed without sedation or general anesthesia in infants and young children.
In lymphedema (see Fig. 14.2), CT scan demonstrates the characteristic reticular pattern and thickening of the subcutaneous tissue [22, 23]. It also provides anatomic localization of the edema which helps differentiate epifascial versus epifascial and subfascial edema. CT venography can also assess increased interstitial fluid formation due to venous hypertension (incompetent valves, venous obstruction). CT may be used to monitor responses to compression therapy in lymphedema through serial measurements of the cross-sectional area and tissue density in the tissue compartments of interest [24].
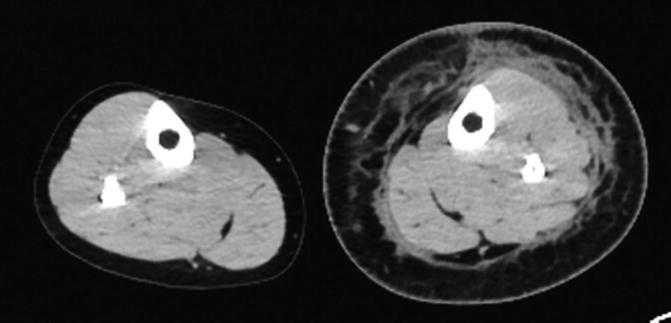

Fig. 14.2
Axial CT scan of the lower extremities in primary lymphedema. Circumferential reticular density is noted within the subcutaneous layer of the calf coalescing in the immediate epifascial plane. There is thickening of skin and subcutaneous fat
Ultrasonography
Ultrasonography (US) is utilized as a noninvasive diagnostic tool for the evaluation of lymphedema. US can be used to rule out the cause of increased interstitial fluid formation due to systemic disease (congestive heart failure, liver disease, renal disease). High-frequency linear-array probes are best for evaluation of superficial tissue. Gray-scale images are routinely obtained in transverse and longitudinal planes. In patients with lymphedema, it shows the thickening of the cutaneous, epifascial tissue compartments, interstitial fluid accumulation and occasionally may allow evaluation of the degree of fibrosis (Fig. 14.3). High frequency sonographic images reveal the characteristic patterns of cutaneous fluid localization in various types of edema [25]. In one study of patients with secondary lymphedema, the relative proportion of fluid and fibrosis identified on sonography correlated well with the clinical findings of soft, medium, hard, or pitting type of edema [26




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







