Key fields
Heterogeneity of a given target disease
Identification and validation of biomarkers and their development as companion diagnostic
Stratification of patient population with the biomarker/endophenotype
Improved genotype-phenotype relationship with information of improved computational medicine
Provide evidence for a better benefit-to-risk ratio and efficiency
Potential of personalized medicine in dermatology
Identification of still healthy individuals with high risk to develop a given disease and the opportunity to act preventively (e.g., atopic dermatitis)
Opportunity for early detection of a disease possibly even before the first symptoms appear (early intervention) and to control them effectively (e.g., psoriasis arthritis)
Better and more precise diagnostic of disease and stratification according to ways for a more adapted therapy (e.g., malignant melanoma)
Prognostic information (e.g., autoinflammatory skin diseases, skin cancers)
Development of more targeted therapies with more efficacies and less side effects (e.g., lupus, malignant melanoma)
Reduce the time, costs, and failure rate of clinical trials for new therapies
Stage adapted therapy decisions and improved treatment algorithms (e.g., skin cancers)
Better monitoring during therapy and more options for alternatives by nonresponders (e.g., skin cancers)
Opportunity for disease-modifying strategy (e.g., skin cancers, atopic dermatitis)
1.2 The Concept and Goals of Personalized Medicine: The Right Patient with the Right Drug at the Right Dose at the Right Time
With the elucidation of the human genome at the beginning of this century [11] followed by the rapid development of bioanalytical high throughput technologies (the so-called omics) [12], a new area in our understanding of the genetic background of many monogenetic but also genetically complex diseases was introduced. Thus, the progress in understanding the genetic and epigenetic complexity for a number of clinical phenotypes has brought substantial information of putative predictive, diagnostic, and prognostic value [13]. The molecular pathways based on the genomic background are increasingly considered for the identification of putative therapeutic targets for some subgroups of patients within one seemingly single clinical phenotype or disease [14]. This kind of stratification of complex and heterogeneous groups of patients [15] ultimately leads to a better definition of disease subgroups where a substantial risk-to-benefit ratio can be afforded in responding patients. In selecting those patients who will respond to a given drug [16] and avoiding to expose unresponsive patients to the same drug with potential side effects will overall increase the effectiveness of a given medial product and decrease the risk for the generation of unnecessary side effects or drug interactions which may induce severe complications and costs.
1.3 The Tools of Personalized Medicine
The biomarkers are the most important tools on which personalized medicine strategies will be based on in the future [17]. The tremendous progress in the different “omics” areas has open an enormous field of investigation for a better understanding of the epi/genetics and the pathophysiological mechanisms leading to complex diseases with a wide clinical and heterogeneous phenotype. These technologies will allow to discover step by step new biomarkers enabling the endophenotype-based stratification of the patients according to elaborated criteria. Besides the aspects of discovery, many efforts will have to be invested in the validation of the biomarkers until they can be considered of clinical use [3]. The identification of relevant biomarkers and their validation can only be reached when they are originated from biobanks implemented by detailed clinical phenotypic information [18]. The huge amount of data which need to be handled in this context is strongly related to sophisticated algorithms integrated in bioinformatics-based system biology [19, 20]. More recently, it also became evident that the microbiome [21] (and the products of the metatranscriptome) must be considered as an important factor in the control of health and diseases. Thus, data from microbiome which is now considered as our second genome, particularly from the skin [22], will be of crucial importance to be included in the strategies mentioned herein. Therefore, establishing and combining (1) high-quality biobanks gathering representative biological samples, (2) high-quality phenotypic information, and (3) state of the art in systems biological tools are considered to be key for the discovery and validation of biomarkers.
1.4 Dissecting the Complex Clinical Phenotypes for Optimized Drug Development and Application
Each disease is characterized by a more or less wide spectrum of individual symptoms building up a complex clinical phenotype but under the heading of one diagnosis. This clinical heterogeneity often mirrors complex pathophysiological mechanisms which may have distinct epi/genetic origins. Similarly, the heterogeneity of the clinical response to the classical treatments includes the risk to apply potent drugs with serious side effects in patients who will not respond to that particular drug [23]. This is one particular and important aspect to which stratified medicine tries to find an answer. The progress in our knowledge on the epi/genetic background and the diversity of the pathophysiological mechanisms leading to complex phenotypes will ultimately lead to a splitting of this heterogeneous phenotype in some more clearly and homogeneously defined subgroup potentially characterized by a given profile of biomarkers and endophenotype (Fig. 1.1). Therefore, it is expected that most diseases will be refined in subgroups according to a biomarker-based molecular taxonomy [24, 25]. Besides the genomic and epigenomic information as well as the biochemical and immunological pathways, a number of other information will be gathered and integrated such as the metatranscriptome [26], diet, lifestyle, exposure to environmental factors, and many others in order to better understand the individual profile of each patient in the hope to switch from the current attempt to cure diseases towards future prevention approaches. The current approach of personalized medicine is requesting the interaction of numerous stakeholders facing a number of challenges. The success is tightly dependent on the progress in the identification of relevant biomarkers [27] enabling us to stratify complex phenotypes and to identify those patients with the highest response to a given drug with the lowest possible side effects. Finally it should also be mentioned that personalized medicine generates substantial ethical [28] and socioeconomic issues [29, 30] which cannot be addressed in this short review but are of real concern at all levels.
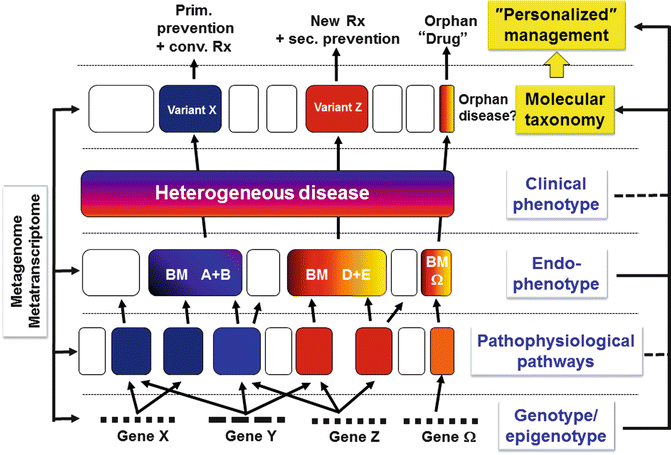

Fig. 1.1
Endophenotype-based stratification of heterogeneous clinical phenotypes into variants and the consequences for personalized management
1.5 Conclusion and Outlook
As a consequence of tremendous progress in biomedical research and diagnostic technologies, an endophenotype-based stratification of complex clinical phenotypes will allow to better address the patient population which will have the highest benefit of targeted therapy with a significantly improved safety profile. The combination of several biomarkers with different predictive and prognostic values [31] will enable to optimize the management of hitherto lethal or debilitating diseases. Thus, a kind of refinement with increasingly complex biomarker profiles will emerge, ultimately reaching the level of truly individualized medicine. As an obvious consequence of a modern endophenotype-based strategy, it will be possible to intervene in a pathologic process before the symptoms become apparent or before it has caused irreversible damages, i.e., disease-modifying strategies will become a reality [9, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








