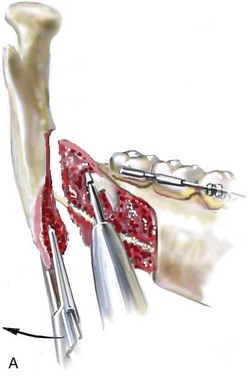
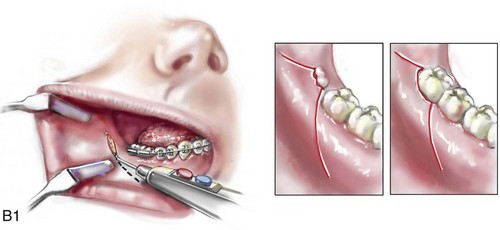
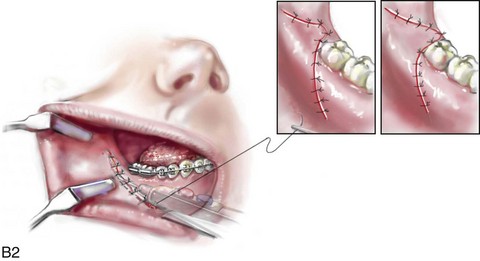
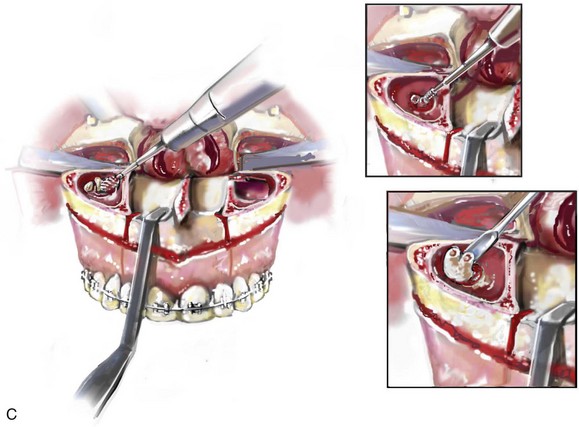
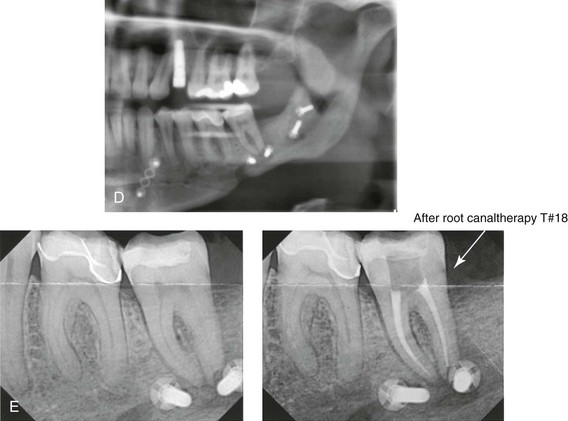
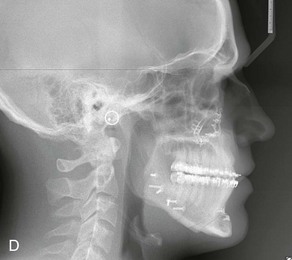
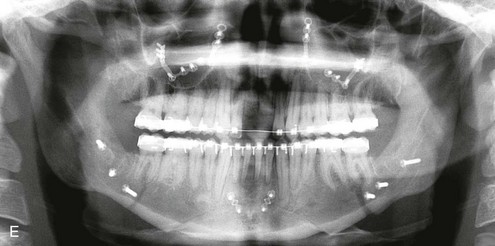
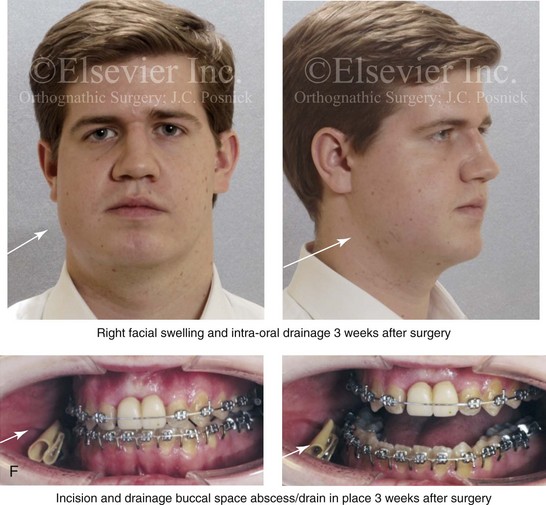
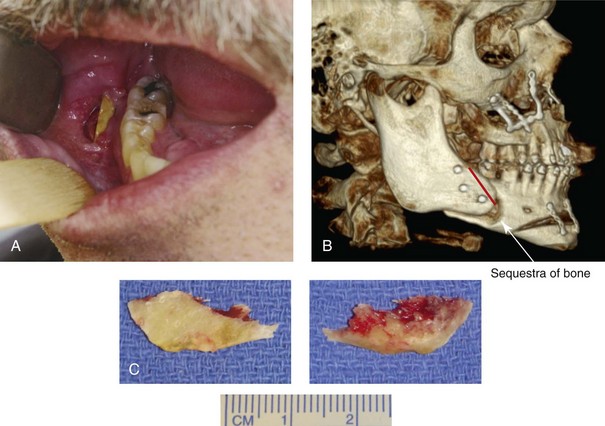
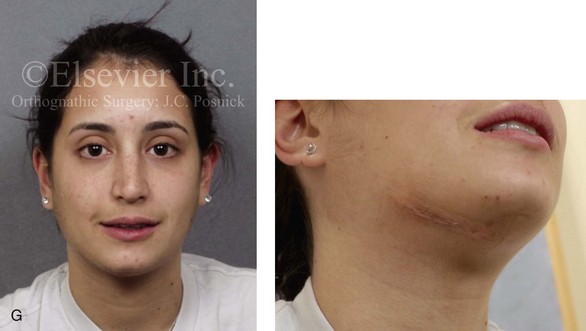
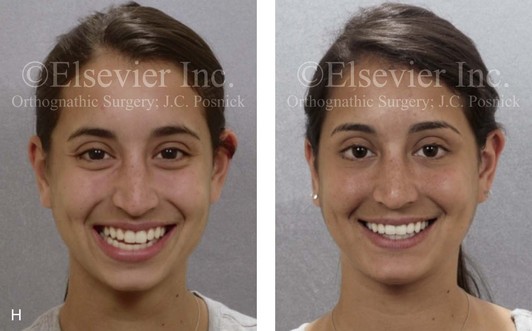
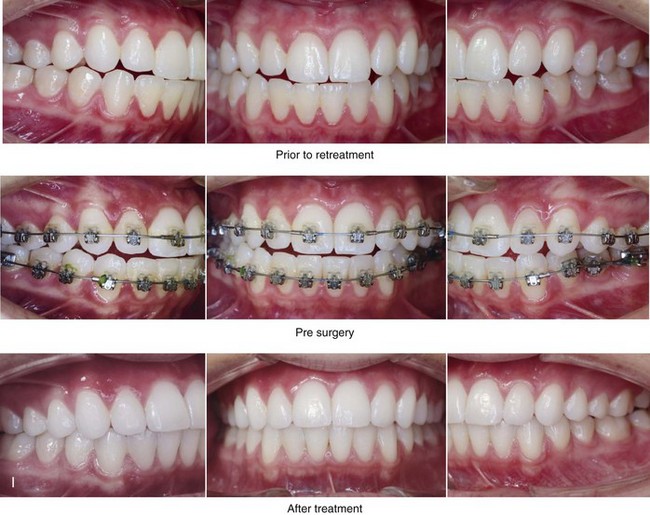
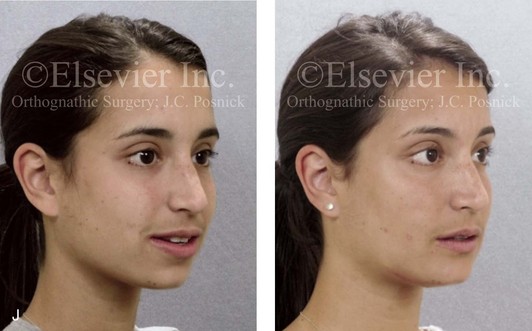
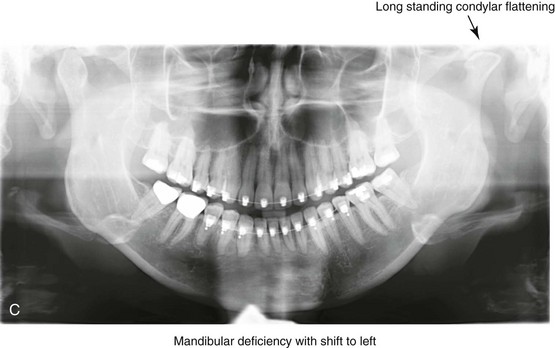
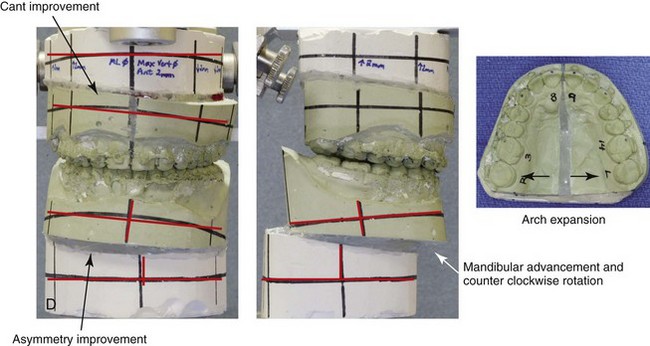
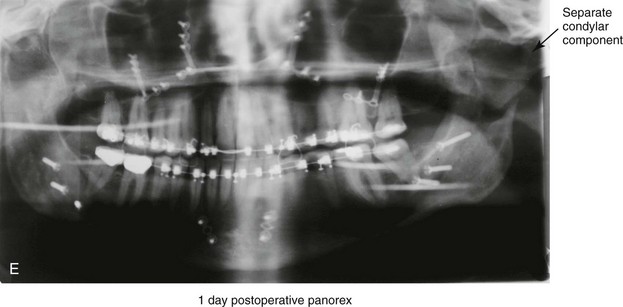
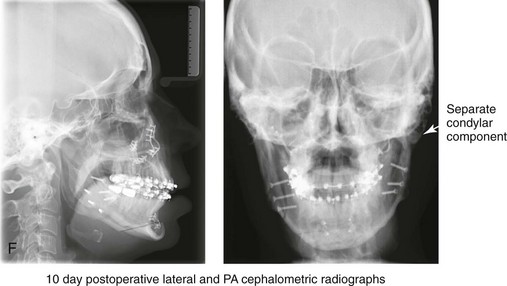
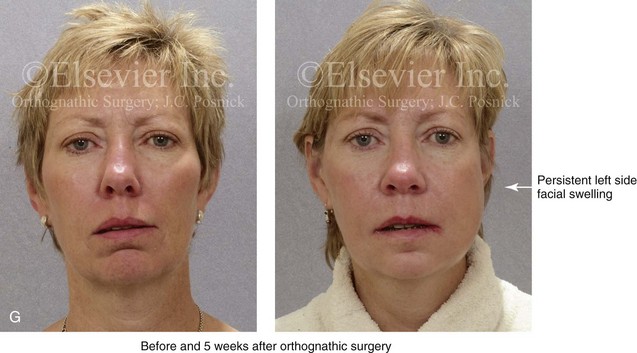
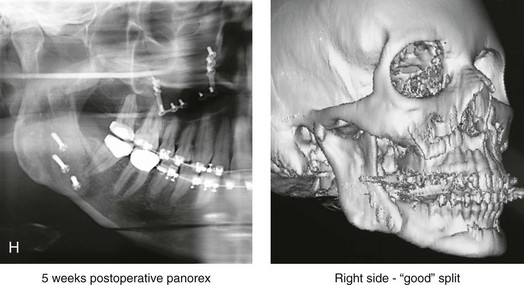
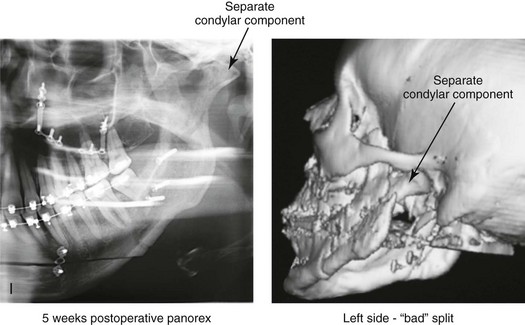
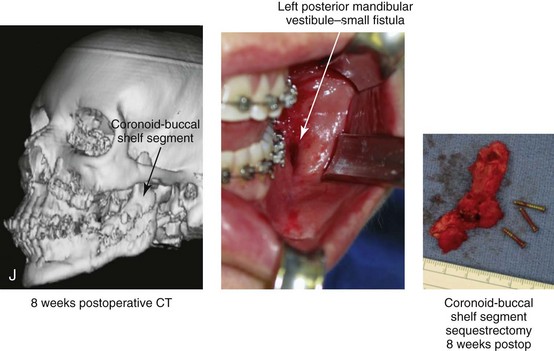
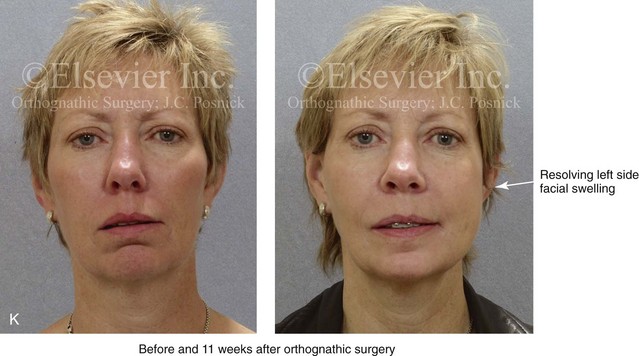
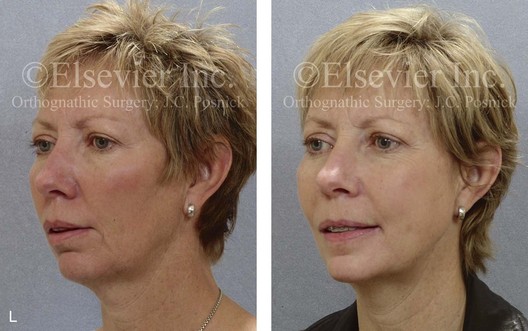
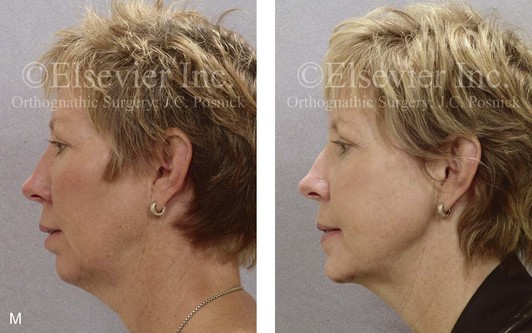
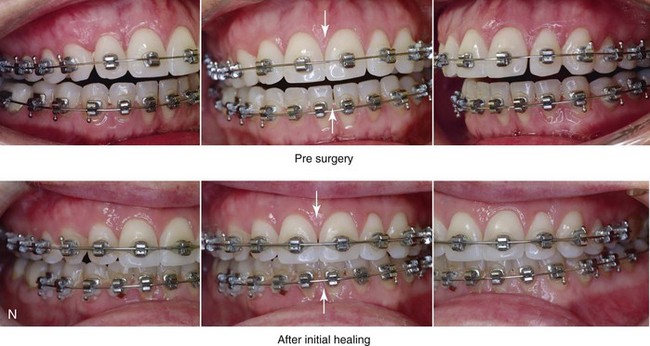
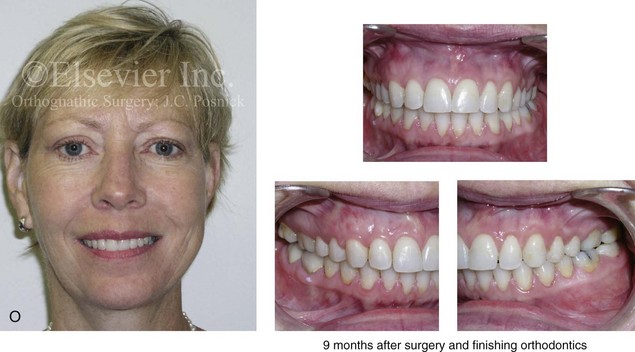
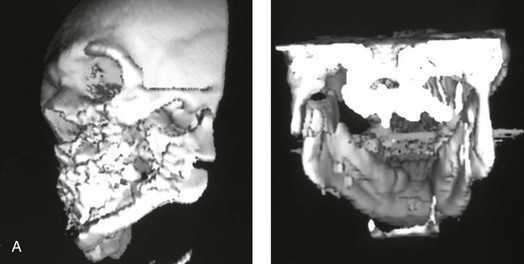
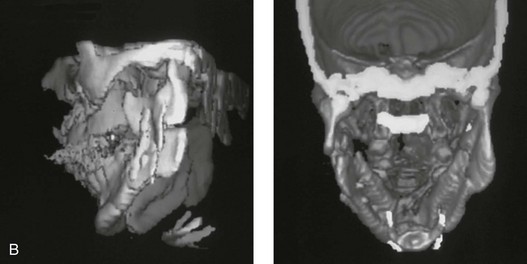
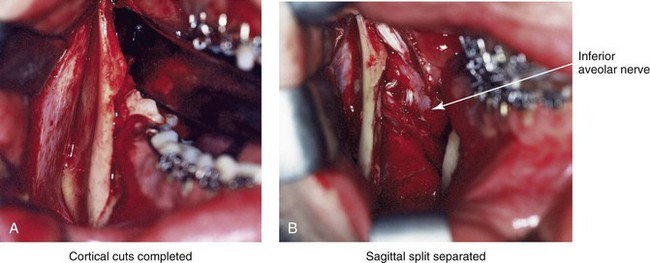
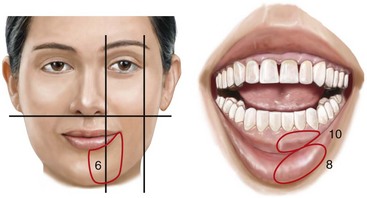
16 No matter how accurate the diagnosis and how meticulous the surgeon, complications will occur after orthognathic procedures.* Alternatively, the relative safety of orthognathic procedures has been confirmed by a number of retrospective reviews.13,32,84,93,152,223,245,254,315,353 Panula and colleagues reviewed maxillary and mandibular osteotomy patients at their institution (N = 655) and found only one serious complication (intraoperative bleed). A prospective study of Le Fort I operations by Kramer and colleagues (N = 1000) found a 6.4% incidence of complications that ranged from extensive bleeding (1.1%) to sensory loss in the distribution of the infraorbital nerve.173 A review by Chow and colleagues of a consecutive series of orthognathic patients (N = 1294) documented a relatively high complication rate (9.7%)61; this included mostly manageable “postoperative infections” (7.4%), with no serious or rare events recorded. A large series of orthognathic patients (N = 2049) was reviewed by Van de Perre and colleagues346; those authors identified one patient death and 44 other patients with either primary or secondary complications. For middle-aged adults who are considering orthognathic surgery, additional medical risk factors may include cardiovascular disease; obesity or increased body mass index; obstructive sleep apnea; diabetes; pulmonary disease; smoking history; the potential for deep vein thrombosis or pulmonary embolus; and osteoporosis or the use of bisphosphonate treatment (see Chapter 25).30,50,172 Evaluation by the patient’s primary care physician (and, in many cases, by other medical specialists) and appropriate laboratory work (e.g., electrocardiography, chest radiography, chemistries, hematology, thyroid tests, coagulation studies) help to identify areas of concern. The adult patient with an elevated body mass index who is undergoing surgery is at greater than average risk for intraoperative and postoperative complications. The presence of obstructive sleep apnea in the patient with a jaw deformity should be considered and ruled out (see Chapter 26). Specific patient factors that may impair wound healing include diabetes mellitus, smoking, bisphosphonate treatment for osteoporosis, poor nutritional status, and chronic glucocorticoid exposure. Type II diabetes mellitus is frequently a comorbidity of obesity and increased body mass index. Alpha and colleagues documented a 66% incidence of postoperative infections among diabetic patients undergoing maxillary or mandibular osteotomies, despite adequately controlled glucose levels.9 The inhalation of nicotine via cigarettes affects both pulmonary function and wound healing.72,75,95,264,137,149,176 Smoking or nicotine ingestion has been shown to delay the chondrogenic phase of bone (osteotomy) healing and to decrease the circulation of surgically elevated flaps, thereby resulting in impaired healing after wound closure. Cigarette smoke contains nicotine, carbon monoxide, and hydrogen cyanide; each of these chemicals has been shown to impair wound healing by producing relative tissue hypoxia. Nicotine is a vasoconstrictor that diminishes tissue oxygenation, predisposes the individual to microvessel thrombosis (i.e., increased platelet adhesion and direct endothelial cell damage), and diminishes cell proliferation and function. Carbon monoxide reduces oxygen-carrying capacity, and hydrogen cyanide inhibits oxidative metabolism and oxygen transport. These consequences of cigarette smoke tissue ischemia are most evident during healing after surgical procedures, when tissue circulation is at risk for interruption (e.g., after microvascular tissue transfer, during the placement of free [non-pedicled] bone grafts). A number of clinical studies have documented increased morbidity in patients who smoke and later undergo head and neck reconstructive and aesthetic procedures. To minimize postoperative wound difficulties and anesthesia-related pulmonary complications, patients should be counseled to refrain from smoking for at least 6 to 8 weeks preoperatively and for 2 to 4 weeks postoperatively. A serum or urine nicotine concentration can be obtained before surgery to confirm patient compliance. It is known that bisphosphonate treatment for osteoporosis can affect osteoclast activity and that it may negatively impact successful bone healing. Current guidelines concerning bisphosphonate treatment continue to evolve and should be followed when planning jaw or dentoalveolar procedures.1,85,125,198,209,214,215,220–222,248,283,285,292,294,357,367 Perioperative risk to the airway and its management should be considered during all phases of surgical care, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation (Fig. 16-1).53,61,76,170,175,250 The basic clinical preoperative airway assessment should include 1) measurements of mandibular range of motion (i.e., vertical opening and protrusion); neck mobility 2) Mallampati classification 3) the ratio of the patient’s height to the thyromental distance 4) neck circumference and 5) body mass index. A Mallampati classification of III or IV or a ratio of height to thyromental distance of more than 23.5 is a strong indicator of a difficult airway with conventional direct laryngoscopy. A neck circumference of more than 17 inches in men is a risk factor for obstructive sleep apnea. Individuals who are scheduled to undergo orthognathic surgery may have special medical risk factors that include baseline malformations or syndromes and associated abnormalities that carry the potential for difficult airway control (see Chapter 11). In the high-risk patient, advance discussion with the anesthesia team to arrange for an anesthesiologist who is experienced with difficult away management and who is skilled with awake fiber-optic intubation may be indicated (see Fig. 16-1) ( Figure 16-1 Perioperative risk to the airway and its management should be considered during all phases of orthognathic surgery, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation. Individuals who are scheduled for orthognathic surgery and who are recognized to be at high risk for airway compromise should be reviewed in advance with the anesthesia team to arrange for special needs. Three patient examples in which these issues came into play are shown, including an adult with Noonan syndrome, a teenage girl with Treacher Collins syndrome, and a teenager with Klippel–Feil anomaly. Each of these patients also has the potential for cervical spine anomalies with limited neck range of motion. The risk for spinal cord injury should also be evaluated before orthognathic surgery. Malignant hyperpyrexia involves an abnormality of muscle metabolism of genetic origin that manifests as widespread skeletal muscle contraction, hypercatabolism, and hyperthermia upon exposure to volatile anesthetic agents or succinylcholine.192,223,365 It is a serious condition that requires prompt diagnosis and aggressive management. It may occur during an orthognathic procedure with a typical initial intraoperative presentation of tachycardia and noticeably warm skin. Decisions about the timing of extubation after surgery should be made by clinicians on the basis of extubation criteria and patient-specific risk factors.159,376 In general, the surgeon is present at the time of extubation in case either emergency tracheostomy or reintubation is required. Our preference is to extubate the patient in the operating room when feasible but only when specific parameters are met. For the patient with an ongoing difficult airway, a more delayed approach to extubation maybe taken. The risk of inadequate ventilation after extubation is highest when pre-extubation parameters are not adequately met and when excess sedation remains (see Chapter 11). Spontaneous leg compartment syndrome has been reported as a complication after orthognathic surgery.204 The occurrence is thought to be the result of a drug interaction between anesthetic or postoperative medications or the effect of antidepressive drugs that are superimposed on chronic mild exercise-induced compartment syndrome. Death is recognized as an exceedingly rare complication related to orthognathic procedures. Of the numerous large case series published, only one death has been described; this was reported by Van de Perre and colleagues from a series of 2049 patients.210 It occurred in a 17-year-old male patient who was described as being slightly mentally challenged and who underwent bimaxillary osteotomies. Six hours postoperatively, a cardiac arrest occurred, and attempts to resuscitate the patient failed. The cause was thought to be preexisting cardiomyopathy. A death was also reported by Waack and colleagues as part of a limited series of 63 consecutive Le Fort I osteotomies.216 The death occurred in a healthy 15-year-old female patient on the first postoperative day as a result of unknown causes. As part of the presurgical assessment, the evaluation of the patient’s cervical spine is essential. Congenital malformations, previous trauma, and arthritis are occasional etiologies of cervical spine problems in the orthognathic patient. Patients with specific syndromes (e.g., Klippel–Feil anomaly, hemifacial microsomia, Treacher Collins syndrome, Apert syndrome) are at higher risk for a cervical spine malformation that may be undiagnosed at presentation to the orthognathic surgeon (see Fig. 16-1). Referral for evaluation by an orthopedic spine surgeon, a neurosurgeon and a geneticist as well as appropriate radiographic studies (e.g., magnetic resonance imaging, computed tomography) are carried out when indicated. Controlled positioning with limited flexion, extension, and rotation at the time of intubation, intraoperatively, and during recovery may be required. In some cases, intraoperative neuro-monitoring (e.g., motor and sensory) is useful to confirm minimal spinal compression during surgery. To minimize patient and family disappointment after surgery, an informal (or, in some cases, a more rigorous) psychosocial assessment is conducted.24,36,73,81,167,304,317 All parties involved must accept the risks and limitations of the planned surgical procedures. Unfortunately, neither the preoperative psychosocial assessment nor the individual’s biologic wound-healing ability is completely predictable. A small percentage of patients will perceive an unfavorable surgical outcome, even when the clinicians feel that the results are at least satisfactory. Others will not accept a complication, even though the risks were fully explained in advance and all precautions were taken. Body dysmorphic disorder is a medical condition that can be difficult to recognize before surgery but that will routinely result in postoperative patient dissatisfaction. It is estimated that 7% to 15% of individuals who pursue cosmetic surgery have body dysmorphic disorder (see Chapter 7). Recognizing when a patient who is seeking orthognathic surgery has dental needs that go beyond standard orthodontics is essential to the achievement of an optimal outcome (see Chapter 5). If complex dental restorations and periodontal management are necessary, then planning should coordinate care for these needs at the beginning of treatment (see Chapter 6). This is especially true for the individual with a cleft lip and palate or a craniofacial syndrome (see Chapters 28 through 34). It is also true for the adult who has suffered dental and periodontal sequelae from an uncorrected dentofacial deformity or previous treatment (e.g., orthodontics, restorative care) (see Chapter 25). The orthognathic surgeon should evaluate the patient’s baseline head and neck functions, including speech, swallowing, mastication, neck and mandibular range of motion, breathing, hearing, vision, cognition, and psychosocial competence. Any of these functions may be negatively affected by the presenting maxillofacial deformity or malformation and influenced (either favorably or unfavorably) by the treatment being contemplated.94,113,139,163,228,232,311,313,320,377 A candid discussion with the patient and the family about the potential impact of the planned procedures on any of these functions should take place before treatment. Limiting risks and complications requires an informed patient and family, coordinated collaborative comprehensive clinical care, an experienced and focused surgeon, meticulous planning, a dedicated operating room team, effective postoperative hospital care, and appropriate outpatient management. The operating room team includes the surgeon and his or her assistants, the anesthesiologist and his or her assistants, the operating room personnel (e.g., scrub nurse/technician, circulating nurse), and the full complement of sterilized and organized instrumentation (e.g., inventory, sanitation and sterilization personnel). The instrumentation includes power saws and drills, with their associated bits and blades; handheld instruments; and fixation hardware such as plates and screws. Specific medications, sutures, and dressing materials are also required. The confirmation of the prepared operating room team, equipment, instrumentation, and medications should be undertaken before the patient enters the operating room. The anesthesiologist and the surgeon should discuss the patient’s airway and his or her intraoperative and postoperative needs before entering the operating room (see Chapter 11). After the equipment, instrumentation, and medications are set up and checked and the team is briefed, the patient may enter the operating room with the best opportunity for success. The effects of orthognathic surgery on temporomandibular joint function are often specific to the patient and cannot be fully predicted.11,82,138,139,156,247 Many clinical studies report improvement in temporomandibular joint symptoms in a majority of patients after successful orthodontics and orthognathic surgery, whereas some of the study patients show deterioration. The favorable effects of orthognathic surgery for patients with preexisting temporomandibular joint symptoms may result from the improved occlusion, the corrected facial height and horizontal projection, and the reduced emotional stress (or the placebo effect). Varied degrees of documented radiographic condylar remodeling after orthodontics and orthognathic surgery have been reported in some patients. Interestingly, TMJ radiographic findings do not necessarily correlate with symptomatic temporomandibular disorders (see Chapters 9 and 37). Clinical studies have now documented no increased risk of unfavorable or so-called “bad splits” when SROs are performed in patients with impacted third molars as compared with those without third molars.90,218,268,276,348 Doucet and colleagues laid this argument to rest with their prospective cohort study that looked at the effects of the presence or absence of third molars during SRO on 1) the frequency of unfavorable fractures 2) the degree of entrapment of the IAN in the proximal segment and 3) the procedural time required to move the teeth.89 The study included patients who were scheduled to undergo SRO to correct a dentofacial deformity. Study patients were divided into two subgroups: Group I was made up of those with an impacted mandibular wisdom tooth at the time of SRO (n = 331; mean age, 19.6 years); Group II included those without a mandibular wisdom tooth at the time of SRO (n = 346; mean age, 30.4 years). The overall incidence of unfavorable fractures was 3.1% (21 out of 677), with frequencies of 2.4% (8 out of 331) in Group I and 3.8% (13 out of 346) in Group II. Interestingly, the rate of IAN entrapment in the proximal segment was significantly lower in Group I (37.2%) than in Group II (46.5%). The removal of third molars at the time of SRO increased procedural time by only 1.7 minutes. When the removal of erupted or partially erupted mandibular wisdom teeth is carried out at the time of SRO, the standard vestibular incision for SRO is altered to achieve access to the erupted wisdom teeth and then for watertight wound closure (Fig. 16-2, B) (also see Chapter 15). In review, when considering evidence-based medicine, the routine practice of the removal of wisdom teeth before SRO can no longer be justified. When removing impacted maxillary wisdom teeth, access through the floor of the antrum after down-fracture at the time of Le Fort I osteotomy represents this author’s usual approach (Fig. 16-2, C). The exception is when carrying out segmental osteotomies in a cleft maxilla in conjunction with extensive horizontal advancement of the lesser segment. In these cases, the removal of the wisdom tooth in the lesser segment may significantly diminish bone volume with potential compromise of stabilization and healing. Metal plates and screws have been used to stabilize fractures and osteotomies in the maxillofacial region for more than 40 years.8,9,12,39,46,47,104,131,151,164,225,281,336 The metals that were initially used were stainless steel and vitalium; these were followed by titanium and its alloys. There is both laboratory and clinical evidence of the tissue compatibility and the high corrosion resistance of titanium and its alloys in the human body. In 1991, the Strasbourg Osteosynthesis Research Group recommended that “the removal of a non-functional plate is desirable provided that the procedure does not cause undue risk to the patient.”32A,355A During earlier years, those who were advocating the routine removal of fixation devices after stable bone union felt that the implant had significant potential to cause problems, so its removal was considered preventative. Today, clinicians agree that the medical risk and financial costs incurred by the routine removal of asymptomatic fixation hardware cannot be justified. It is currently standard practice to remove fixation plates and screws only when clinically indicated. Becelli and colleagues completed a retrospective analysis of complications of bicortical screws used for sagittal split ramus osteotomy (SSRO) fixation.22 The authors reviewed a consecutive series of patients (N = 241) who were undergoing sagittal ramus osteotomies (N = 482) for skeletal Class III deformities. Complications related to bicortical titanium screws were observed at 3% of the osteotomy sites (Fig. 16-3). The hardware was eventually removed in these patients (15 out of 482), without long-term consequences. Figure 16-3 An 18-year-old with a bilateral cleft lip and palate jaw deformity underwent cleft–orthognathic surgery including maxillary Le Fort I osteotomy in three segments and interpositional iliac bone grafting. Healing was uncomplicated, with the achievement of the planned improvements. One year after surgery, the patient returned with a complaint of a painless ridge in the left buccal vestibule of the maxilla. Examination confirmed an exposed titanium plate and screw. There were no associated symptoms or signs of infection. During an office procedure, the exposed plate was sectioned with a wire cutter and removed. Healing was uneventful. The use of self-reinforced biodegradable bone plates and screws has been advocated for use in orthognathic surgery by a limited number of surgeons throughout the world.92,96,127,130,155,165,178,297,325,344,345 Although this type of fixation is appealing in theory, a greater incidence of postoperative complications has been found with resorbable fixation devices as compared with titanium. Furthermore, no advantages in the achievement of improved skeletal stability have been documented with the use of biodegradable hardware. Turvey and colleagues reported about 70 patients who were undergoing 194 osteotomies of the maxilla or the mandible and who were stabilized with self-reinforced polylactate bone plates or screws of similar size and configuration to those of titanium systems.344 The placement of the devices was accomplished transorally and transfacially in a way that was consistent with the osteotomy location. Maxillomandibular elastics were used for each patient after surgery to control the position of the jaws. Three out of the 70 patients (4%) experienced immediate postoperative loosening of the resorbable bone screws. Each of the patients required a return to the operating room for more rigid stabilization at the involved osteotomy site. Although all patients eventually achieved acceptable occlusion, a number of fixation sequelae occurred during the healing phase, including prolonged mobility of the maxilla; sterile abscess requiring debridement; swelling with sterile fistula tract formation; and intranasal inflammation requiring fixation removal at the time of secondary septoplasty. Although the authors demonstrated that biodegradable plates and screws could be used for orthognathic surgery, there was no advantage when this was compared with titanium fixation devices, and the complication rate is increased. In a second study, Turvey and colleagues reported about 69 patients who were undergoing sagittal split ramus osteotomies for mandibular advancement.345 Thirty-four of these patients underwent fixation with self-reinforced biodegradable screws, whereas 35 patients underwent fixation with 2-mm titanium screws. Postoperatively, one of the patients with biodegradable screws required a return to the operating room for improved fixation. Again, no advantage could be demonstrated with the use of biodegradable fixation, whereas a degree of diminished levels of stabilization and increased complications were shown to be present. Ahn and colleagues completed a study to evaluate the complications of resorbable versus nonresorbable plate and screw fixation in orthognathic surgery.3 Patients were enrolled in the prospective study (N = 272). Titanium plates and screws were used at the osteotomy sites in Group I (N = 152), and resorbable plates and screws were used at the osteotomy sites in Group II (N = 120). In the 152 patients with titanium plates and screws, there was a complication rate of 8.6% (N = 13). Of the 120 patients with resorbable plates and screws, 18.3% (N = 22) developed complications. There was a greater degree of postoperative infections and malocclusions and a trend toward relapse observed in patients with resorbable plate and screw fixation. Three of the patients with resorbable plate and screw fixation required reoperation for residual malocclusion as compared with zero in the titanium group. Infection after orthognathic surgery is considered by most to be uncommon. However, a review of the literature indicates a wide range in the reported incidence of this complication.* A critical review of these studies provides insight into the true incidence of infection, clinical pitfalls to consider, and methods to use to best limit these sequelae. All transoral wounds must be considered “clean-contaminated wounds,” with an anticipated theoretical infection rate of as high as 15%.352 It is said that this incidence of infection in a clean-contaminated site can be reduced to as low as 1% with good surgical technique and the appropriate use of prophylactic antibiotics. The question remains of how to best provide this type of antibiotic coverage for the orthognathic surgery patient. According to the infectious disease literature, antibiotic prophylaxis should provide adequate drug levels in the tissue before, during, and for the shortest possible time after surgery to provide a reduced infection rate; be an effective agent against the bacteria that are most likely to cause the infection; and be bactericidal while at the same time being the least toxic agent available. Spaey and colleagues reported a 4% prevalence rate of infection after orthognathic procedures in their cohort.312 Among the infections that were sustained, 92% occurred at the SSRO site, 1% occurred at the Le Fort I osteotomy site, and only 0.5% involved the chin osteotomy location (Figs. 16-4 through 16-7). In their pilot study group, after completion of an SSRO, the authors placed a drain through the mandibular vestibule mucosa incision; this was removed the following morning. The prophylactic antibiotic regimen that was initially used did not have a drug level in place while the drain remained in or after its removal. Because of the high infection rate observed in the pilot study, the researchers changed the protocol to no longer involve the placement of a drain; to achieve a more meticulous mucosal wound closure; and to extend the time of prophylactic antibiotic use to 5 days after surgery. As a result, the infection rate at the SSRO site was dramatically decreased. The authors concluded that, in addition to an extended use of prophylactic antibiotics, the achievement of a sealed vestibular wound at the SSRO is essential to limit the infection rate. Figure 16-4 A woman in her early 30s with a primary mandibular deficiency growth pattern underwent a comprehensive orthodontic and surgical correction. This required the removal of dental compensation, including opening upper bicuspid extraction spaces for dental implants. Surgery included bilateral sagittal split ramus osteotomies, an osseous genioplasty, and anterior neck soft-tissue procedures. In-hospital and early at-home convalescence proceeded without incident. Five weeks after surgery, the patient returned to a more normal diet, to regular physical activities, and to her orthodontist’s care. Two months after surgery, pain in the left mandibular second molar region with chewing was persistent. The tooth was also tender to percussion. A periapical radiograph revealed the proximity of a bicortical fixation screw to the distal root, with possible apical root resorption. The removal of the involved screw was offered. However, the patient chose root canal therapy followed by the relief of pain and the resolution of percussion tenderness. A, Profile and lateral cephalometric views before treatment. B, Oblique facial views before and after treatment. C, Pretreatment and posttreatment lateral cephalometric radiographs. D, Left side Panorex image 2 months after surgery, with left mandibular second molar pain with chewing. E, A periapical radiograph is shown before and after successful root canal therapy (see Fig. 19-8). Figure 16-5 A 23-year-old man with a developmental jaw deformity and malocclusion was referred for surgical evaluation. He had also recently sustained trauma to the maxillary anterior region that involved a loss of the central incisors. The jaw deformity was characterized by maxillary deficiency in combination with relative mandibular excess. The patient agreed to a comprehensive surgical and dental rehabilitative approach. He also had lifelong difficulty breathing through his nose. After periodontal and restorative evaluations, the patient underwent orthodontic (dental) decompensation. Surgery included maxillary Le Fort I osteotomy in segments (arch expansion, horizontal advancement, and cant correction); bilateral sagittal split ramus osteotomies (asymmetry correction); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. In-hospital and initial at-home convalescence proceeded without difficulty. Three weeks after surgery, increased swelling and tenderness along the right ramus region were appreciated, and intraoral drainage through the lateral ramus incision was recognized. The patient underwent exploration with Penrose drain placement as an office procedure under local anesthesia, and he was restarted on antibiotics. The drain was removed 1 week later, and the 10-day course of oral antibiotics was completed. Healing at all osteotomy sites remained on track. Five weeks after surgery, the patient returned to a more regular diet and physical activity without delay. The orthodontic appliances were removed 6 months after surgery. A, Presurgical facial and occlusal views with orthodontics in progress. B, Profile and lateral cephalometric views before surgery. C, Articulated dental casts that indicate analytic model planning. D, Three week postoperative lateral cephalometric radiograph. E, Three week postoperative Panorex radiograph. F, Facial and occlusal views 3 weeks after surgery at the time of persistent right facial swelling and intraoral drainage. The patient underwent exploration and Penrose drain placement. Figure 16-6 A 50-year-old man with a short face growth pattern and obstructive sleep apnea underwent orthognathic surgery, including maxillary Le Fort I osteotomy; bilateral sagittal split osteotomies of the mandible; and an osseous genioplasty. Interpositional allogenic grafting was required in the maxilla and chin regions. At the 2-week postoperative visit, partial dehiscence of the right mandibular vestibular wound was recognized. The distal superior aspect of the proximal segment was visualized through the wound. There was minimal associated facial swelling and no abscess or fluid collection. In the office setting, a rongeur was used to remove the exposed bone, and the patient was restarted on antibiotics. The wound spontaneously closed over the next 10 days. Healing at the osteotomy site remained on track, and there was no long-term sequelae. (Note: If the inferior borders had been more closely aligned before the bicortical screw fixation, then dehiscence with the loss of the bone fragment may not have occurred.) A, Intraoral view of the right mandibular vestibular wound at 10 days after surgery with dehiscence and exposure of the superior aspect of the proximal segment. B, Computed tomography scan demonstrating the portion of the buccal shelf and the proximal segment that was resected with the rongeur. C, Specimen removed with a rongeur. Figure 16-7 A 20-year-old college student with an asymmetric mandibular excess growth pattern (Hemi-mandibular elongation) arrived for surgical evaluation. She had previously undergone orthodontic attempts to neutralize the occlusion without success. She agreed to a combined orthodontic and surgical approach. With the removal of orthodontic (dental) compensation, surgery was carried out. The procedures included bilateral sagittal split ramus osteotomies (asymmetry correction) and osseous genioplasty (horizontal advancement). By 5 weeks after surgery, postoperative swelling had generally subsided. A painless “lymph node” was palpable in the right submandibular region. It was not fixed to the skin or bone. Three months after surgery, what appeared to be a localized lymph node became tender with erythema and then with drainage through the skin. Cultures were carried out and showed no specific growth. An infectious disease consult was obtained, and Gram staining showed gram-positive rods. The patient was placed on amoxicillin and then Augmentin. The localized infection involved intermittent drainage but without a connection down to the bone. Four months after surgery, the patient agreed to undergo excision of the soft-tissue mass with primary closure and the reinstituting of antibiotics. Histopathology was unremarkable, and the cultures remained negative. The wound healed without complications. A, Facial and occlusal views with orthodontics in progress. B, Articulated dental casts that indicate analytic model planning. C, Facial views with smile before and 5 weeks after surgery. D, Lateral cephalometric radiographs before and 5 weeks after surgery. E, Panorex radiographs before and 5 weeks after surgery. F, Right submandibular localized infection demonstrated by 3 months after surgery. G, Four months after surgery, the soft-tissue infection was excised and sent to the pathology and bacteriology departments. H, Frontal views with smile before and after successful treatment. I, Occlusal views before retreatment, just before surgery, and then after successful treatment. J, Right oblique facial views before and after successful treatment. K, Right profile views before and after successful treatment. In a randomized, double-blind study, Ruggles and colleagues tested two different antibiotic regimens in patients undergoing orthognathic surgery.284 Both groups received preoperative and intraoperative antibiotics, whereas only one group continued 48 hours of postoperative coverage. The group that received no postoperative antibiotics had a 15% infection rate, whereas no infections were found in the group with extended antibiotic coverage. Danda and colleagues completed a prospective study to evaluate the use of prophylactic antibiotics among patients who were undergoing orthognathic surgery.78 The study patients (N = 150) were divided into two groups. Group I (N = 75) received a single dose of antibiotic prophylaxis. Group II (N = 75) received a full day of antibiotic prophylaxis. In Group I, 9.3% of patients (N = 7) developed infection, whereas only 2.6% (N = 2) of Group II patients did. The results indicate a clinically significant increased rate of infection with single-dose antibiotic prophylaxis as compared with a full day of antibiotic prophylaxis. Kublefelt and colleagues reported on smoking as a significant risk factor for infection after orthognathic surgery.176A This was a retrospective cohort study of individuals (n = 286) who were undergoing one-jaw orthognathic surgery (i.e., bilateral SSRO [BSSRO] or Le Fort I osteotomy) during a 7-year period. All patients received a 1-week course of antibiotics. Patients ranged in age from 17 to 56.5 years (mean, 34.8 years). The overall infection rate was 9.1%. The only statistically significant identified risk factor for infection in these study groups was smoking history. The incidence of infection was 14.4% among smokers and 7.0% among non-smokers. The site of infection was primarily the ramus region followed by the Le Fort I osteotomy region. Interestingly, six cases of actinomycosis after orthognathic procedures have been reported.216,293 Ozaki and colleagues reported a case of actinomycosis that occurred in the left submandibular area after SSRO.249 The infection resolved after 2 months of antibiotic therapy. Manikandban and colleagues reviewed the incidence and consequence of bur breakage within the surgical wound during orthognathic surgery.210 The authors found that surgical bur breakage was not uncommon (5 out of 76 patients; 6.6%). They concluded that bur retrieval was not always advisable, because collateral damage to the hard and soft tissues may occur in the process. They recommended radiographic confirmation immediately after surgery and then clinical follow up for at least 1 year to confirm that no foreign body reaction or infection has occurred (Fig. 16-8). Figure 16-8 Individuals who are undergoing orthognathic surgery may experience intraoperative bur breakage. It is also not uncommon for orthodontic brackets, hooks, or ligatures to loosen with the potential for loss in the surgical field. Because of the small size of the lost objects within deep wounds, retrieval is not always advisable or possible. These foreign bodies can be visualized radiographically. Clinical follow up for at least 1 year is recommended to confirm that no foreign body reaction or infection has occurred. A Panorex radiograph is shown before and after orthognathic surgery. An orthodontic hook was lost in the right mandibular vestibular wound and can be seen on the Panorex radiograph. No negative sequelae resulted. When completing an SRO, an unfavorable fracture (i.e., a “bad split”) can occur.23,42,139,162,206,243,334 The definition of a bad split varies from clinician to clinician. This author reserves the term bad split only for those SROs in which the condyle either remains with the distal segment, thus requiring a separate osteotomy (i.e., third piece), or shatters into a separate component (i.e., third piece) on its own (Fig. 16-9). Other less than ideal splits may include the following: 1) a separate fracture or piece of the buccal plate 2) a separate fracture or piece of the lingual plate posterior to the second molar; or 3) a separate fracture or piece of the coronoid process. All three of these splits are generally manageable without alteration of the expected postoperative recovery. When the buccal plate separates from the proximal segment, the fragment is either removed or additional plate and screw fixation is used to secure it. When the lingual plate separates from the distal segment, no additional fixation is generally required. The lingual bone fragment is either removed or left in place. When the coronoid process separates from the proximal segment, it is usually removed to limit the chance of ankylosis during the healing process. Figure 16-9 A woman in her mid 40s was referred for the surgical evaluation of asymmetric mandibular deficiency with secondary deformities of the maxilla. A longstanding flattening of the left condylar head with a loss of posterior facial height was felt to be responsible. There were no current symptoms of temporomandibular disorders, and the condyle was felt to be stable. The patient had an asymmetric Class II excess overjet anterior open-bite malocclusion. An orthodontic and surgical approach was selected. With orthodontic (dental) decompensation complete, surgery included maxillary Le Fort I osteotomy in segments (arch expansion and cant improvement); bilateral sagittal split ramus osteotomies (minimal horizontal advancement and asymmetry improvement); osseous genioplasty (horizontal advancement); redo septoplasty; and neck liposuction. During surgery, a “bad split” was appreciated on the left side. Nevertheless, the ramus osteotomy was secured with three bicortical screws. Radiographs the day after surgery confirmed a separate condylar component in the left ramus region. Posteroanterior and lateral cephalometric and Panorex radiographs were again taken 10 days after surgery, and these were suggestive of adequate segment approximation. At 5 weeks after surgery, left lateral mandibular facial swelling with mild tenderness persisted. At 8 weeks after surgery, a left posterior mandibular vestibular fistula was appreciated. In the operating room, the wound was opened to remove a buccal shelf and coronoid fragment as well as the fixation screws. Cultures confirmed Escherichia coli, Enterobacter, alpha-hemolytic Streptococcus, and Candida. Oral antibiotics were initiated, including clindamycin, ciprofloxacin, and Diflucan. At 11 weeks after surgery, there was a functional occlusion with good vertical mouth opening and a mild shift to the left. At 9 months after surgery, facial morphology and occlusion remained acceptable, and the orthodontic appliances were removed. A, Facial and occlusal views with orthodontics in progress. B, Profile and lateral cephalometric views before surgery. C, Panorex radiograph before surgery that indicates longstanding left condylar head flattening with an intact cortical rim. There is mandibular deficiency and a shift to the left. D, Articulated dental casts that indicate analytic model planning. E, Panorex radiograph 1 day after surgery suggests a separate condylar component from the left ramus. F, Lateral and posteroanterior cephalometric radiographs taken 10 days after surgery indicate the same. G, Facial views before and 5 weeks after surgery. Persistent left side facial swelling is appreciated. H, Panorex and computed tomography scan views of the right ramus 5 weeks after surgery. I, Panorex and computed tomography scan views of the left ramus region 5 weeks after surgery confirm a “bad split” that demonstrates the expected anatomy. J, Left ramus computed tomography scan, left intraoral vestibular view, and sequestrectomy specimen with removed fixation screws. K, Frontal views in repose before and 11 weeks after surgery. L, Left facial oblique views before and then 11 weeks after surgery. M, Left profile views before and 11 weeks after surgery. N, Occlusal views before surgery and then 11 weeks postoperatively. O, Nine months after the completion of treatment, frontal facial and occlusal views demonstrate the results. When the condyle separates as a third piece, intraoperative decisions will affect the postoperative recovery and occlusion (see Fig. 16-9). From the perspective of the occlusion, if the condyle or the medial pole remains with the distal segment, it will be as if no osteotomy on the ipsilateral side of the mandible was carried out. If no further action is taken, an intraoperative malocclusion should be recognized. To fully complete the ramus osteotomy, the surgeon must then separate (i.e., osteotomize) the condyle from the distal segment (i.e., create a third piece). The osteotomy should be carried out, with as much ramus left on the condylar segment (i.e., the third piece) as possible. Even when this type of bad split occurs, unless significant mandibular advancement is required for the correction of the jaw deformity, the successful healing of the segments will generally occur (see Figs. 16-9 and 16-10). Figure 16-10 A teenager with a cleft jaw deformity underwent orthognathic surgery that included a maxillary Le Fort I osteotomy in segments; bilateral sagittal split osteotomies of the mandible; and an osseous genioplasty. The sagittal split on the left side separated unfavorably. The proximal segment includes a condyle coronoid upper ramus component; an angle ramus component; and a buccal shelf component. The three components of the proximal segment were secured together and fixed to the distal segment with a combination of titanium plates and screws. Fortunately, the reduction was adequate, so healing occurred with favorable facial aesthetics, occlusion, and temporomandibular joint function. A and B, Three-dimensional computed tomography scan views of a “bad split” fixed into acceptable alignment during orthognathic surgery. If the condyle separates (i.e., if a third piece is created) “high up,” the result is similar to that of a high condylar neck fracture. There will not be a stable posterior stop unless the surgeon is able to carry out plate and screw fixation of the condylar component (i.e., the third piece) to the rest of the proximal segment. This may not be practical, and it will depend on the morphology of the condylar piece. The incidence of postoperative malocclusion will also be dependent on the directional change planned for the distal mandible. If the mandible is to be advanced a significant amount, there is a greater probability that the minimally stabilized condylar segment (i.e., the third piece) will not heal in an ideal location relative to the residual proximal and distal segments. Alternatively, if the mandible is to remain relatively neutral or is set back, there is a higher probability that the condylar segment will heal to the residual proximal and distal segments in a favorable way (see Fig. 16-9). As long as healing is associated with adequate mobility (i.e., mouth opening), then any residual malocclusion can typically be managed 6 to 12 months later with redo SROs, if desired (see Chapter 35). The occurrence of a bad split is greatly reduced by keeping the medial osteotomy “short (not to far back) and low” (close to the occlusal surface of the mandibular molars) (Fig. 16-11). Using this technique a bad split as described should be a rare event (see Chapter 15). Figure 16-11 Illustration of this author’s preferred “short and low” medial osteotomy of the sagittal split to minimize an incidence of a “bad split.” Veras and colleagues evaluated risk factors and the functional and radiographic long-term results of a bad split.350 They also completed detailed temporomandibular joint examinations on patients with bad splits. Interestingly, they did not find the simultaneous extraction of a mandibular third molar in conjunction with an SSRO to be a risk factor for the development of a bad split. This is consistent with the research reported by Kriwalsky and colleagues,174 Precious and colleagues,268 and others (see discussion earlier in this chapter). Facial sensibility of the area affected by either an SSRO or a chin osteotomy occurs through the mandibular nerve, which enters the mandibular foramen at the medial surface of the ramus.* This area is adjacent to and in front of the inferior alveolar artery and vein and just inferior to the occlusal plane of the mandibular molar teeth. The mandibular nerve becomes the IAN, and it runs with its vessels within the canal and supplies sensation to the three molars and the two premolar teeth. It then divides into two terminal branches. The incisive branch supplies sensation to the canine and incisor teeth, whereas the mental nerve exits from the foramen to supply sensibility to the chin, the lower lip mucosa and skin, and the adjacent gingiva. Damage to the IAN during either the ramus osteotomies or when completing an oblique osteotomy of the chin may occur. During SSRO of the mandible, the IAN can be damaged by a bur on a rotary drill, a saw blade on a reciprocating saw, an osteotome, or during the placement of plate and screw fixation (Fig. 16-12). Figure 16-12 Intraoperative views of a right sagittal split ramus osteotomy. The demonstration of cortical cuts completed A, before and B, after the splitting (separation) and mobilization of the segments. The inferior alveolar nerve is visualized in the surgical field. Posnick and colleagues completed a study to clarify normal chin, lower lip, and gingival sensibility in adolescents and then compared the normal values with sensibility measurements in three distinct groups of adolescents 1 year after these individuals had undergone mandibular osteotomy.261,263 One hundred fifteen subjects (230 mental nerves) were included in the study and divided into four groups. The first group included 67 individuals (Group I: n = 134 nerves; mean age, 18 years) who were undergoing orthodontic treatment; none had undergone orthognathic surgery. This group served as controls to determine the normal sensibility values of the chin, the lower lip, and the gingiva in adolescents. The remaining three groups were made up of adolescents who had previously undergone BSSRO of the mandible (Group II: n = 14 nerves; mean age, 19 years), osteoplastic genioplasty (Group III: n = 40 nerves; mean age, 19 years), or a combination of BSSRO and osteoplastic genioplasty (Group IV: n = 42 nerves; mean age, 19 years). Testing of Groups II, III, and IV was performed 1 year after the patients had undergone the indicated mandibular procedures. At the time of the 1-year postoperative examination, each patient was also asked to detail subjective impressions of current altered sensibility in the mandibular vestibular mucosa, the gingiva, the lower lip, and the chin region. The subjective questioning and objective testing were carried out by an occupational therapist who was trained in facial sensibility testing and familiar with the surgical procedures carried out. Fixed and reproducible coordinates in the area corresponding to the mental nerve were used for objective testing (Fig. 16-13). Three sensory modalities were tested at each coordinate for each involved nerve. Static two-point discrimination was measured with a MacKinnon–Dellon Disk-Criminator (Richardson Productions, Inc, Frankfort, Ill); vibratory thresholds were determined with a biothesiometer; and cutaneous pressure thresholds were measured with a set of Semmes–Weinstein monofilaments. Sensibility values at 1 year after operation for Groups II, III, and IV were compared with the mean normal control values established for Group I. A subjective awareness of alterations in the sensibility of the chin, lip, or gingiva region 1 year after BSSRO was reported in 2 patients in Group II (29%), in 2 patients in Group III (10%), and in 14 patients in Group IV (67%). Interestingly, only 2 of these 18 patients considered the residual sensory loss problematic. The mean objective sensibility values of the three sensory modalities tested were highest among patients from Group IV. Significant differences were found only between the mean two-point discrimination of Group IV patients as compared with the control group in the chin skin area. Thus, 1 year after BSSRO, only 29% of patients had residual subjective awareness of sensory alteration along the distribution of the mental nerve. After an osseous genioplasty, there was only a 10% incidence of long-term subjective diminished sensibility. One year after a combination of BSSRO and osteoplastic genioplasty, 67% of patients experienced varying degrees of residual subjective loss of sensation in the chin and lip region. Figure 16-13 Illustration of sites for sensibility testing in the inferior alveolar and mental nerve distribution. In a study carried out by Posnick and colleagues, coordinates 6, 8, and 10 located within each of the circled areas were tested bilaterally. Espelan and colleagues completed a 3-year postoperative survey of patients who had undergone SSRO (N = 583 subjects) and found that that 36.8% of the study patients reported impaired sensation over the long term.96A Westermark and colleagues completed a retrospective study of patients who had undergone SSRO and concluded that nearly 100% of the patients experienced impaired sensation and sensory function.358 This is also consistent with the findings of Essick and colleagues97 and Teerijoki-Oksa and colleagues.332,333 The exact reasons why, over time, most patients experience only a degree of simple loss of sensation—whereas some continue to experience active sensations that are not normally present and an even smaller percentage of those with active sensations subjectively describe them as uncomfortable or painful (dysesthesia)—are not known. Zuniga and colleagues reported a 5% incidence of dysesthesia of the IAN after SSRO.380,381 Turvey reported a 5.5% incidence of IAN lacerations at the time of SSRO.343 Van Merkesteyn and colleagues documented a visible injury in the IAN at the time of SSRO in 7 out of 124 patients (6%).347 The term traumatic neuroma is used to describe the formation of a bulbous mass that develops at the end of a proximal nerve stump after partial or complete nerve transection. The lesion represents an exaggerated relative hyperplasia (i.e., healing) response to a nerve injury. Neuroma symptoms range from paresthesia to severe paroxysmal or persistent pain. Despite the incidence of partial or complete transection of the IAN at the time of SSRO, few cases of traumatic neuroma have been documented. This is likely because the healing transected nerve remains deep in the medullary cavity of the mandible. It is known that a healing nerve stump within bone is rarely painful. The few reported cases of neuroma after SSRO have confirmed the location to be within the soft tissues along the inferior border of the mandible.120,121 Essick and colleagues completed a clinical trial that determined that the magnitude and duration of the patient-reported burden of altered sensation after SSRO was lessened when facial sensory retraining exercises were performed in conjunction with standard mouth-opening exercises as compared with the group of patients who performed only mouth-opening exercises.97 The authors defined the term burden as the person’s reporting of subjective impressions of numbness or unusual sensations (i.e., dysesthesia) in the face, the perioral region, and or oral region. One interpretation of the study findings is that patients who perform facial sensory retraining become more introspective with regard to their altered sensation, which leads to a greater acceptance of their sensory loss by 6 months after surgery. Consistent with this interpretation was the finding that sensory retrained patients initially viewed numbness and decreased sensitivity as more problematic early during recovery but as less problematic later during recovery than did the group of patients who did not undergo retraining.258 The retraining sessions were held at 1 week, 1 month, and 3 months after surgery. The levels of instruction for the sensory retraining were designed to increasingly challenge patients’ sensory discrimination abilities in a manner similar to that of the early and late phases of sensory retraining commonly used after injuries to the sensory nerves of the hand.120,121 Through sensory retraining, individuals learn to discriminate moving touch from non-moving touch; the orientation of moving touch; and the direction of moving touch (see Chapter 11). Poort and colleagues completed a literature review of methods that are used to test for sensory loss and recovery after IAN injury and recommended that clinicians and researchers use the following two measurement tools: 1) a light touch test with Semmes–Wienstein monofilaments for grading sensation; and 2) a visual analog scale–based questionnaire to evaluate the individual’s subjective sensibility.261A If clinicians were to adopt consistent measuring techniques, improved communication with regard to sensory loss and level of recovery after interventions would likely follow. A dilemma that surgeons continue to face is what to do in the operating room when the laceration of the IAN is witnessed. The question raised asks if there is any significant long-term advantage for the patient to undergo either primary or delayed micro repair of the lacerated IAN. Although the concept of the immediate repair of a witnessed IAN transection is appealing, the practicality of this maneuver and the patient’s long-term improved results (i.e., greater sensory return and decreased dysesthesia) have not been statistically confirmed. The increased operative time and the potential risks of losing focus on the primary orthognathic objectives must also be considered. Tay and colleagues reported four cases of immediate micro-neural repair of an IAN that was transected during SSRO.334 This represented 4 out of 260 (2%) SSROs completed at their center. One of the four patients who sustained IAN laceration was apparently lost to follow up. Interestingly, the location of IAN transection in the remaining three study patients was at the anterior and inferior aspect of the osteotomy along the buccal shelf between the first and second molars; this is a recognized “danger zone” for IAN transection during SSRO. When there is a moderate to severe degree of mandibular hypoplasia with the need for buccal shelf extension of the proximal segment to achieve successful approximation of the bone after advancement, this risk may increase. In the study by Tay and colleagues, the three study patients underwent immediate repair of the IAN (i.e., neurorrhaphy) by a trained microsurgeon and then received follow for 1 year.334 The microsurgeon’s technique included the complete surgical release of the nerve from the mandibular foramen to the mental foramen followed by approximation and repair with the use of 6-0 microfilament suture under microscope magnification. The increased time of the surgery was approximately 3 to 4 hours. Although the authors felt favorable regarding the outcomes of their patients, the level of sensory return that was documented was not proven to be significantly better than expected after the simple approximation of the nerve endings within the ramus of the mandible. The incidence of injury to the lingual nerve during SSRO is not precisely known, but it is assumed to be rare.35,132,145,147,279,289–291,327 Becelli and colleagues completed a retrospective analysis of complications after SSRO and found a 0.6% rate of lingual nerve dysfunction among 482 osteotomy patients during the first postoperative week. The authors documented complete resolution of this injury after 12 months of follow up in the study group. Interestingly, lingual nerve injury at the time of the removal of mandibular wisdom teeth as an isolated procedure is also estimated to be in the same range (i.e., <1%).260 When a lingual nerve injury does occur with SSRO, it likely occurs during subperiosteal dissection along the medial ramus; when using a rotary drill (e.g., with a Lindeman bur); or when using the reciprocating saw (e.g., during a medial ramus cortical cut). It may also occur with use of the electrocautery to control bleeding, during screw fixation, or when closing the wound if the lingual nerve is caught within the suture tie (Fig. 16-14). Figure 16-14 A completed right sagittal split ramus osteotomy. The separation of the segments demonstrates both the inferior alveolar and lingual nerves. The lingual nerve is only occasionally visualized in the surgical field. Pogrel and Hung Le completed a cadaver study that demonstrated that different modalities (e.g., sharp scalpel laceration, fissure bur injury, retraction stretch injury) produced different types of nerve injury, both clinically and histologically.261 The clinical recovery of the injured nerve is believed to be primarily related to the type of injury sustained. For this reason, the treatment recommended should also vary in accordance with the type of injury sustained. Data reveal that 57% of lingual nerve injuries were unknown to the surgeon at the time; therefore, the type of injury could not be accurately ascertained.86 Unfortunately, many of the published lingual nerve injury studies are retrospective case series or questionnaire surveys.260
Complications Associated with Orthognathic Surgery
General Considerations
Medical Risk Factors
Wound-Healing Risk Factors
Perioperative Airway Risk Factors
![]() Video 5). After the nasotracheal tube is in place, the surgeon secures it and assists with its management during the operation (see Chapter 15). Complications associated with endotracheal tubes that are placed for orthognathic procedures have included herniation of the airway tube cuff leading to occlusion of the tube lumen; inadvertent surgical sectioning of the endotracheal tube; and the occurrence of contact granuloma of the vocal cords.62,249 Acute intraoperative or postoperative pulmonary edema, postoperative apnea, pneumomediastinum, and pneumothorax have all been reported as complications associated with anesthesia (see Chapter 11).14,33,34,56,91,117,122,153,217,233,245,259,318,321
Video 5). After the nasotracheal tube is in place, the surgeon secures it and assists with its management during the operation (see Chapter 15). Complications associated with endotracheal tubes that are placed for orthognathic procedures have included herniation of the airway tube cuff leading to occlusion of the tube lumen; inadvertent surgical sectioning of the endotracheal tube; and the occurrence of contact granuloma of the vocal cords.62,249 Acute intraoperative or postoperative pulmonary edema, postoperative apnea, pneumomediastinum, and pneumothorax have all been reported as complications associated with anesthesia (see Chapter 11).14,33,34,56,91,117,122,153,217,233,245,259,318,321
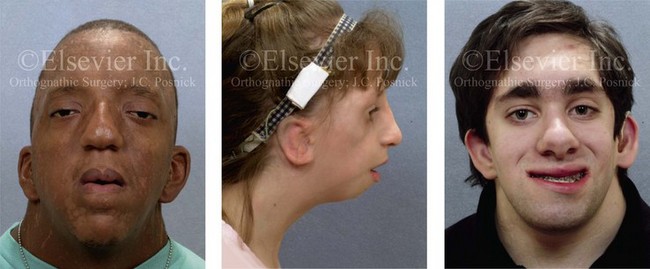
Cervical Spine Risk Factors
Psychosocial Risk Factors
Comprehensive Dental Rehabilitation Needs
Functional Assessment of Head and Neck Structures
Operating Room Team Preparation
Temporomandibular Disorders
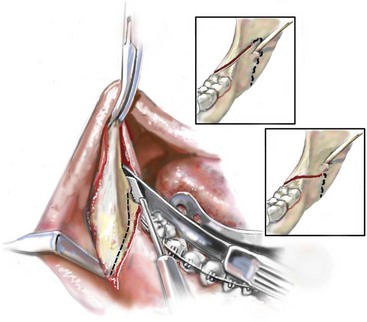
Management of Third Molars
Fixation Considerations
Need to Remove Plates and Screws
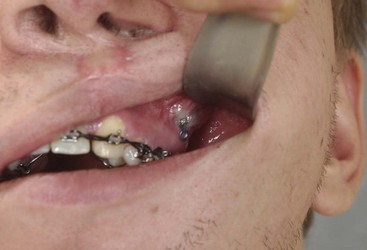
Use of Titanium versus Resorbable Fixation
Infection Associated with Orthognathic Procedures
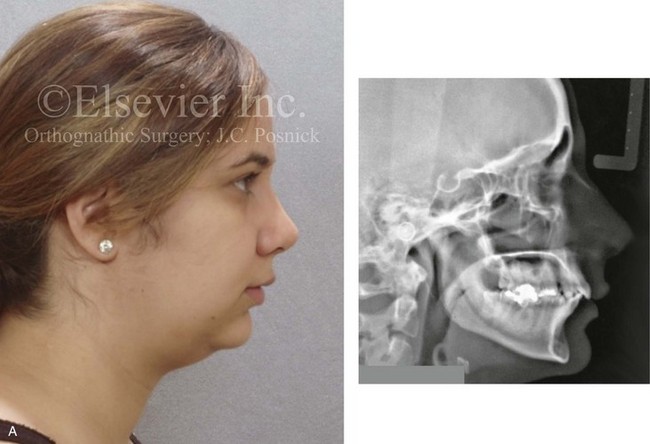

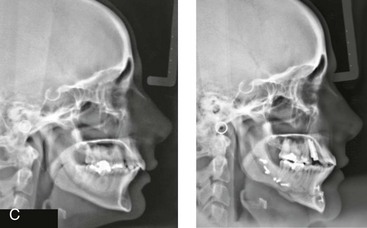
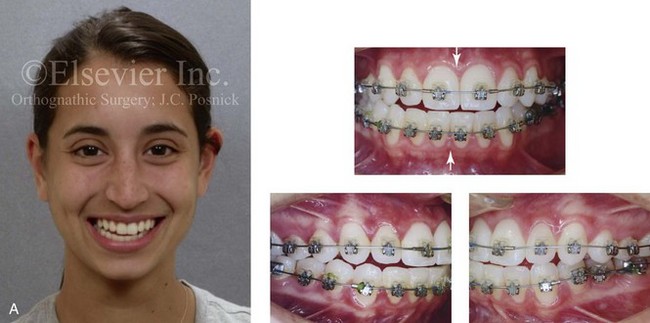


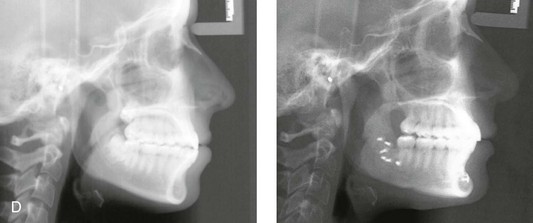
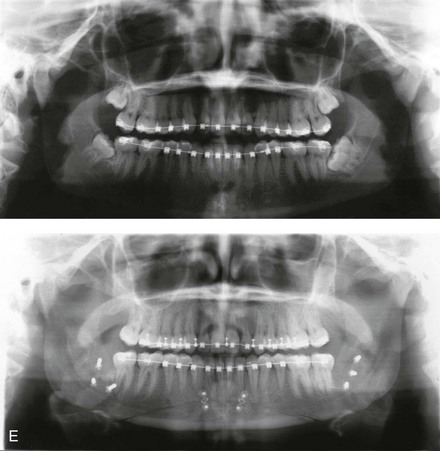
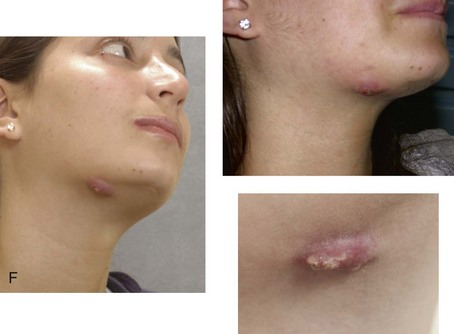
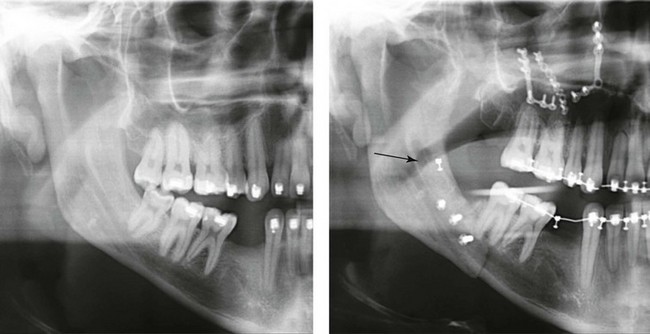
Instrument Breakage: Foreign-Body Reaction
Risks and Complications Specific to Sagittal Split Ramus Osteotomies of the Mandible
Unfavorable Sagittal Split Ramus Osteotomy
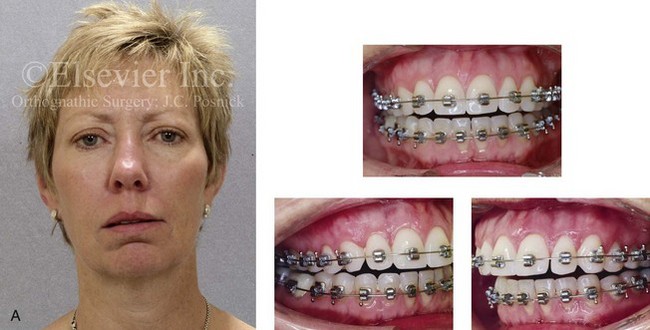
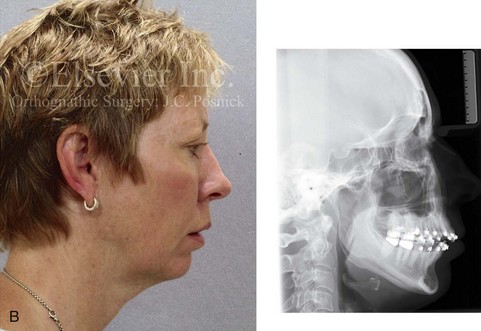
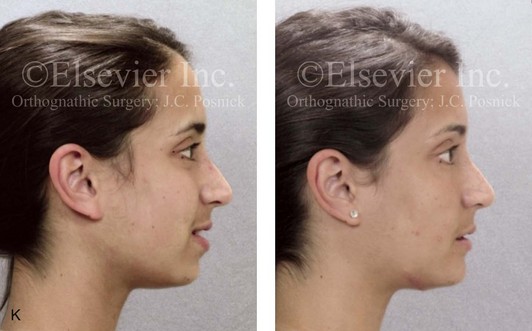
Inferior Alveolar Nerve and Mental Nerve Injury
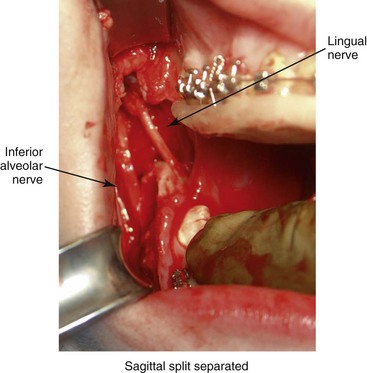
Lingual Nerve Injury
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Complications Associated with Orthognathic Surgery