CHAPTER 8 Complications and Adverse Sequelae of Sclerotherapy
Adverse Sequelae
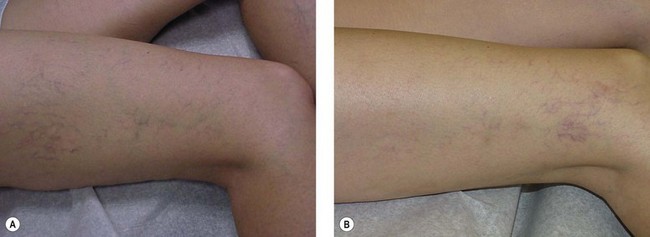
Postsclerotherapy hyperpigmentation
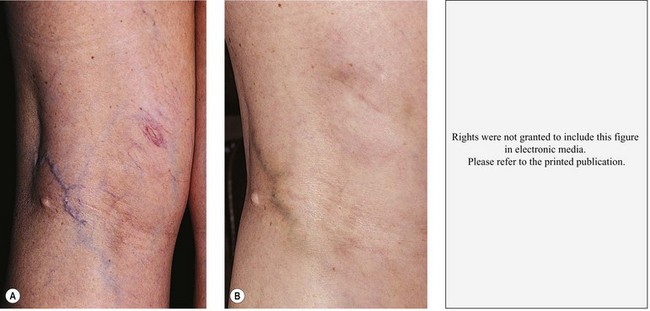
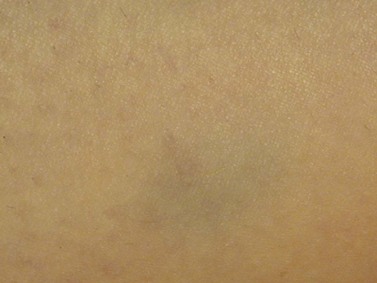
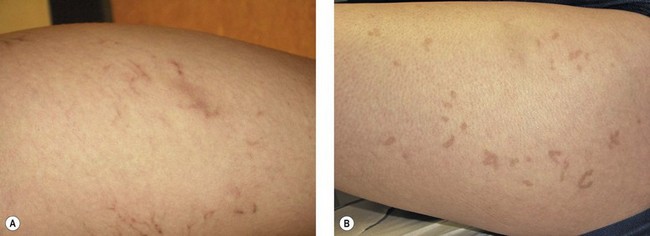
Pigmentation is usually temporary. Physicians report a 1% to 2% incidence of pigmentation persisting after 1 year.1,2 Pigmentation is usually linear along the course of the treated blood vessel. We use the term ghost of the blood vessel to explain to patients that it represents a resolving and not functioning vessel. However, in addition to linear lines of pigmentation, osmotic sclerosing solutions may produce punctate pigmentation at points of injection, which may be related to their mechanism of action through an osmotic gradient that produces maximal osmolality and resultant endothelial destruction at the injection site. In contrast, detergent-type sclerosing solutions destroy the treated vessel for a few centimeters along its length, producing a more linear golden brown color (Figs 8.1 and 8.2). Cutaneous pigmentation is to some degree a relatively common occurrence after sclerotherapy with any sclerosing solution.3 It has been reported in 11% to 80%4–6 of patients treated with sodium tetradecyl sulfate (STS). One study found that a 0.1% concentration of STS resulted in pigmentation in 11% of patients. The incidence of pigmentation with hypertonic saline (HS) has been reported to range from 10% to 30%.6–10 Patients treated with polidocanol (POL) have a reported incidence of pigmentation from 6.7%11,12 to 31%.8,13,14 A 35% incidence has been reported in 7200 patients treated with POL, ethanolamine oleate, or iodine-iodide solution.15 Post-sclerotherapy hyperpigmentation has a reported incidence of 15.7% with Sclerodex (dextrose with sodium chloride),12 and 32% with Sclerodine (iodine and sodium iodide).12 A 2% incidence of hyperpigmentation was reported from one series of patients treated with POL, chromated glycerin (CG), and sodium salicylate.1 A 2%–4% incidence was reported from another series of 102 patients treated with either STS, POL, or CG.16
Between 2003 and 2008, 1187 of our patients underwent sclerotherapy treatment. Of this group, 351 had been treated with foam or liquid STS and were available for follow-up. Thirty-five percent of these patients experienced hyperpigmentation following sclerotherapy. However, hyperpigmentation was graded as minimal to mild. Furthermore, no hyperpigmentation was evident in any patient 1 year after treatment. Of note, the ‘hyperpigmentation’ reported by many patients was actually a post-treatment coagulum.17
Etiologic factors
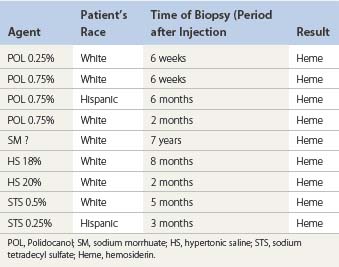
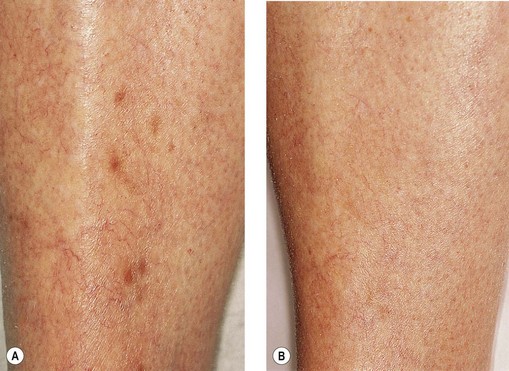
The cause of this pigmentation most likely results from a combination of both postinflammatory hyperpigmentation (incontinence of melanin pigment) and hemosiderin deposition.18–20 However, histologic examination has demonstrated that this pigmentation is caused only by hemosiderin staining of the dermis, irrespective of the type of sclerosing solution used, pigmentation of the patient, or length of time after injection (Fig. 8.3, Table 8.1).21–24 Defects in iron storage and/or transport mechanisms have also been found in a significant number of patients who have developed pigmentation after sclerotherapy.25
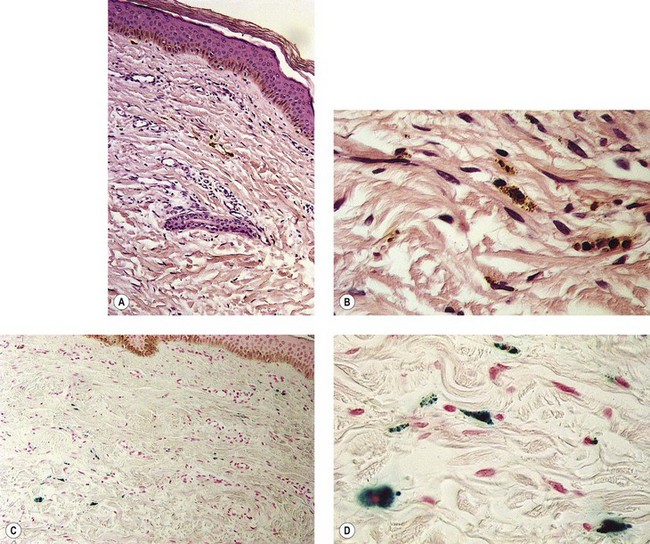
Figure 8.3 Section stained with hematoxylin–eosin taken 6 months after injection with polidocanol 0.75%. Note scattered foci of golden brown pigment. A, Original magnification ×50. B, Perls-stained section from the same patient as in Figure 8.1. Note scattered foci of green-blue granules within siderophages. Original magnification ×200. C, Original magnification ×350. D, Original magnification ×3200.
(From Goldman MP, Kaplan RP, Duffy DM: J Dermatol Surg Oncol 13:547, 1987.)
Hemosiderin deposition occurs predominantly in the superficial dermis, although it may be present in periadnexal and mid-dermal locations, particularly near the ankle. This phenomenon probably occurs when RBCs extravasate into the dermis after the rupture of treated vessels.26 Erythrocyte diapedesis also may occur after inflammation of the vessel and is commonly seen after thrombophlebitis. Perivascular inflammation is presumed to promote degranulation of perivascular mast cells. Released histamine leads to endothelial cell contraction, which results in widening of endothelial gaps through which extravasation of RBCs can occur.27–31 Thus, injecting a sclerosing solution dilates the vessel both directly through pressure generated by the syringe and indirectly through histamine-induced endothelial cell contraction.
Perivascular phagocytosis of RBCs occurs either by intact cells or piecemeal after fragmentation by macrophages.32,33 The intracellular fragments in the macrophage cytoplasm are further compartmentalized into hemoglobin-containing globules. They are referred to as secondary lysosomes. Since hemosiderin is an indigestible residue of hemoglobin degradation, it may appear as aggregates up to 100 µm in diameter.34 Hemosiderin has a variable concentration of these aggregates. Iron concentrations vary from 24% to 36%.35 Iron hydroxide contained in hemosiderin occurs in different forms, with differing amounts of ferritin.36 On unstained tissue it appears golden and is 30% iron by weight. Its elimination from the area through phagocytosis may take years, if it ever occurs.
In addition to being insoluble, hemosiderin may directly affect cellular function. Histologic examination with X-ray fluorescence analysis of patients with varicose ulceration disclosed an elevation of mean iron levels in periulcerated skin.37 The authors speculate that free radical formation resulting from local iron accumulation may cause melanocytic stimulation, thereby augmenting brown pigmentation. Indeed, multiple authors have demonstrated melanin incontinence in the presence of venous stasis, complicated by extravascular RBCs.38–40 Whether melanocytic stimulation plays a role in the early appearance of postsclerotherapy pigmentation is unlikely, but it may contribute to the persistence of pigmentation in certain patients, especially in Fitzpatrick skin types V and VI.
Solution Type and Concentration
The type and concentration of the sclerosing solution affect the degree of endothelial destruction. The extent of endothelial destruction with resulting inflammation and extravasation of RBCs is thought to influence the development of postsclerotherapy hyperpigmentation. The increased incidence of pigmentation with certain concentrations of STS and HS, which produce a greater reaction than POL, confirms this hypothesis.4,41–43 In fact, when excessive concentrations of POL are used to treat telangiectasias (1%), the pigmentation rate is even higher than with 20% HS.44 It is therefore not surprising that sclerosing solutions reported to have the lowest incidence of postsclerotherapy pigmentation – CG,1,41,45–49 glycerin alone,50 and sodium salicylate1,20 – also produce minimal inflammation.
A higher concentration of the same sclerosing solution produces increased inflammation.51 Thus, the inflammatory response after treatment should be kept to a minimum, and sclerosing solutions and concentrations should be altered for each treatment session so that the minimal effective sclerosant concentration is used.
Foam sclerosants are stronger than liquids for an identical concentration. Therefore, when foam is used, special attention should be directed towards reducing the strength or concentration of the agent. This is especially true for treatment of reticular and spider veins.52 Recently published analyses of large numbers of patients treated with foam sclerotherapy estimate the incidence of post-inflammatory hyperpigmentation to be between 10% and 30%.53–55 Furthermore, Alos et al noted that – although the overall incidence of pain with sclerotherapy using 0.5% POL is rare – foam is more often associated with pain than is liquid.56
Technique
Optimal technique consists of limiting pressure into damaged (sclerosed) veins to prevent extravasation of RBCs. To limit the degree of intravascular pressure, larger feeding varices, incompetent varices, and points of high pressure reflux should be treated first. A greater incidence of pigmentation occurs if vessels distal to the saphenofemoral junction (SFJ) are treated before successful closure of the junction, with a decreased incidence of pigmentation when treatment is from proximal to distal.57
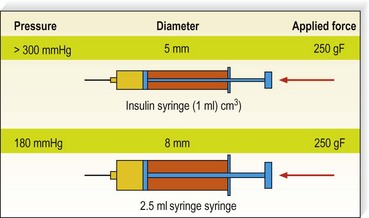
The average piston radius is 8 mm for a 2-mL syringe and 5 mm for a 1-mL syringe. The calculated pressure with an implied force of 250 g is 180 mmHg for a 2-mL syringe and more than 300 mmHg for a 1-mL syringe.58 This is one reason we recommend using a 3-mL syringe for sclerotherapy (Fig. 8.4).
Gravitational and Other Intravascular Pressures
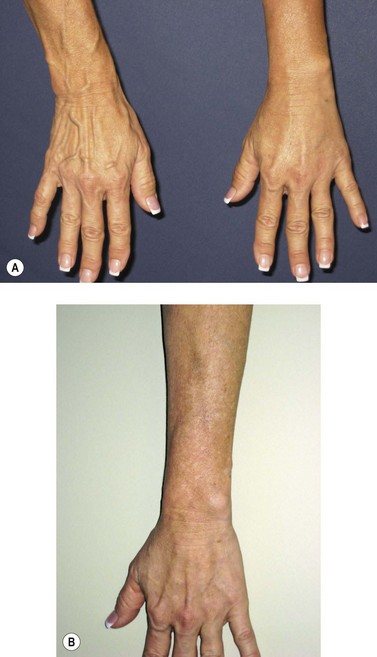
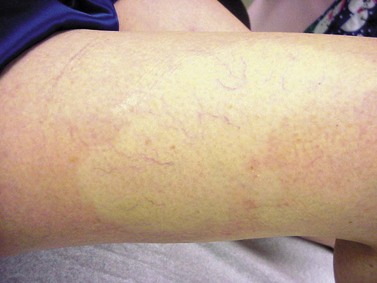
Postsclerotherapy pigmentation appears most commonly in vessels treated below the knee20 but can occur anywhere on the leg, probably as a result of a combination of increased capillary fragility and increased intravascular pressure by gravitational effects in this location. Pigmentation has been observed once in our practice after sclerotherapy treatment of hand veins (Fig. 8.5). Duffy et al59 note that pigmentation did not develop after treating 100 patients with dilated hand veins with either 0.5% STS, 1.5% POL, or 3% POL.
Vessel Diameter
It is commonly observed that telangiectasias that have the maximal incidence of pigmentation are between 0.6 and 1.2 mm in diameter. This could be related to an increased incidence of microthrombi in these vessels. Chatard20 also has observed an increased incidence of pigmentation in the treatment of blue venulectases as opposed to the treatment of red telangiectasias. The reason behind this latter observation is unknown but may be related to vessel diameter, since blue telangiectasias are usually of larger diameter than red telangiectasias. An evaluation of 113 patients treated with sclerotherapy demonstrated pigmentation only rarely in vessels less than 1 mm in diameter.60
Predisposition to Pigmentation
Certain individuals appear to be predisposed to the development of pigmentation through a variety of genetic mechanisms. Pigmentation has been reported as more common and pronounced in patients with dark hair and ‘dark-toned’ skin.18 This may be caused by an increased incidence of postinflammatory hyperpigmentation in patients with these colorings However, Chatard20 reported that pigmentation is unrelated to skin or hair color. We are not aware of the number of Type V and Type VI patients Chartard has treated, but in our experience (RAW, MPG) it is clear that patients with darker skin coloring and Asians do have an increased incidence of post-sclerotherapy pigmentation.
Pigmentation resolves from a gradual resorption of ferritin particles from macrophage digestion. It is hypothesized that the patient’s iron storage and transport mechanisms may influence the rate of clearance of dermal hemosiderin.55 A preliminary study of 16 patients with age-matched controls disclosed that pigmentation developed in patients who had higher serum ferritin levels. Serum ferritin levels correlate with total body iron stores.61 To clarify the relationship between serum ferritin and postsclerotherapy pigmentation, a prospective study of 233 consecutive patients was conducted.62 A linear relationship between the occurrence of pigmentation and pretreatment serum ferritin levels was found at each post-treatment assessment date. This supports the hypothesis that high total body iron stores increase the susceptibility toward hyperpigmentation. However, serum ferritin levels are not an absolute predictor for the development of hyperpigmentation. In a patient with hemochromatosis having a serum ferritin level of 1200 ng/mL, pigmentation did not develop after sclerotherapy of telangiectasia 0.6 mm in diameter with 0.2% STS.63 The explanation may be that this patient’s physician probably used outstanding technique to avoid extravasation of RBCs.
If histamine-induced endothelial contraction promotes extravasation of RBCs or hemosiderin, or both, histamine antagonists should prevent or limit its occurrence. The catecholamines norepinephrine (noradrenaline) and isoproterenol antagonize histamine-induced edema in canine brachial artery preparations.64 Similarly, corticosteroids decrease the size of histamine-induced endothelial gap junctions.65 Terbutaline also inhibits macromolecular leakage from postcapillary venules in hamster femoral veins.66 Cimetidine blocks histamine-induced widening of endothelial gaps in rat femoral veins.67 Therefore, patients who have developed postsclerotherapy pigmentation in past treatments may be pretreated with one or a combination of these medications to block or limit histamine effects.
Vessel fragility may also result in an innate predisposition toward pigmentation. Capillary strength is related to both menstrual cycles and circulating estrogen.68 Decreased capillary strength occurs 3 to 5 days before and 2 days after menses and during ovulation. Fragility has been found to improve with intravenous (IV) and oral administration of conjugated equine estrogen (Premarin) in postmenopausal women.
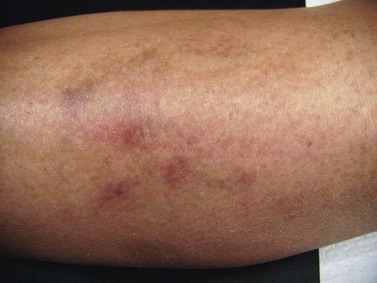
Patients taking minocycline may have an increased risk for postsclerotherapy pigmentation.69,70 This propensity may be related to the inflammatory effects of sclerotherapy. Unlike the golden to deep brown color characteristic of typical sclerotherapy-induced pigmentation, pigmentation associated with minocycline use is most commonly blue–gray (Fig. 8.6). Minocycline, known to have increased tissue distribution due to its lipophilicity, produces pigmentation in a variety of organs and structures.71,72 The most common form of minocycline-related pigmentation appears as bluish-black or bluish-gray macules within acne scars or at other sites of inflammation.10,73–77 Other forms of minocycline-related pigmentation have been described on the skin of the lower legs, including that associated with sites of UV-exposure,78 as well as with sites of pre-existing capillaritis.79 The pigment involved in minocycline hyperpigmentation is most likely a drug metabolite–protein complex chelated with calcium, or an insoluble minocycline–melanin complex.75,78–83 It is hypothesized that minocycline or a metabolite interferes with degradation of hemosiderin through lysosomal disruption, leading to macrophage death and deposition of pigment.77 Recently, four cases of minocycline-related hyperpigmentation involving the subcutaneous fat were reported.84 Histopathologically, tissue exhibited the previously well-described deposition of brown/black, Fontana-Masson and Perls’ positive granules along elastic fibers in the papillary dermis as well as within dermal macrophages located near vessels and eccrine glands. In addition to these dermal findings, tissue from the four patients also showed green–gray, nonrefractile, flocculent globules within macrophages in the subcutaneous tissue. Also, two of the four patients demonstrated the distinctive finding of pigment-related lipomembranous changes. Although further studies are needed to establish a direct relationship, it may be prudent to withhold minocycline therapy in sclerotherapy patients. Of note, successful lightening of minocycline-induced, post-traumatic pigmentary deposition has been described with successive Q-switched Nd:YAG laser treatments.85 Often discontinuation of minocycline will result in spontaneous clearing of minocycline pigmentation within 6 to 12 months.
Postsclerotherapy Coagula
Removal of postsclerotherapy coagula may decrease the incidence of pigmentation. Thrombi to some degree are thought to occur after sclerotherapy of all veins, regardless of size, because of the inability to occlude the vascular lumen completely with external pressure. The persistence of a small vascular lumen, even with maximal external pressure, has been predicted with experimental models of vein wall.86 This has also been directly observed with fiberoptic varicography (Muntlak H, personal communication, 1989).
Persistent thrombi are thought to produce a subacute ‘perivenulitis’ that can persist for months.87–89 The perivenulitis favors extravasation of RBCs through a damaged endothelium or by an increase of the permeability of treated endothelium. In addition, intratissue fixation of hemosiderin may occur.20 This provides a rationale for drainage of all foci of trapped blood 2 to 4 weeks after sclerotherapy. Sometimes blood can be released even 2 months after sclerotherapy.
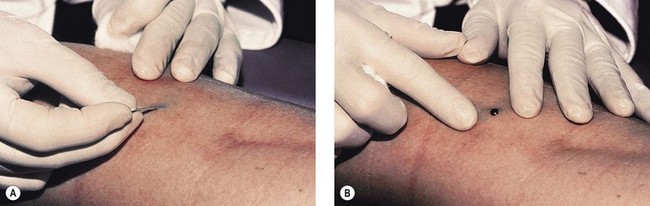
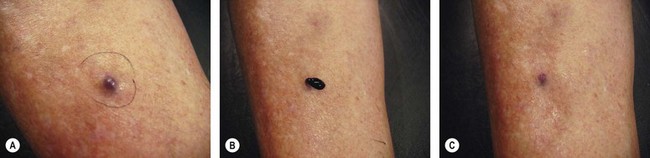
Thrombi are best removed by gentle expression of the liquefied clot through a small incision made with a 21-gauge needle (Figs 8.7 and 8.8). A no. 11 blade or lancet or 18 gauge no core needle have also been recommended by some, but, since this results in a larger incision and often requires pretreatment with a local anesthetic, we favor the more conservative 21-gauge needle approach.
In a multicentered, randomized, controlled study, 101 patients with varicose veins were treated at 1–3 weeks with microthrombectomy in one half of the treated veins.90 Photographs of the sclerotherapy-treated areas were evaluated at 16 weeks. Veins ≤1 mm in diameter had less pigmentation when drained, but veins ≤3 mm did not show any benefit from microthrombectomy.
Perchuk91 raised the possibility of infection occurring from stab incisions. This danger was presumed to be caused by the presence of bacteria in varicose veins – a belief commonly held by physicians 40 to 50 years ago.92,93 However, there have been no reports in the modern medical literature of infections occurring in patients treated with stab incisions into postsclerotherapy clots. This problem has not occurred in our practice, in which this procedure is used routinely.
Duration
Despite therapeutic attempts, pigmentation often lasts from 6 to 12 months.23 Rarely, pigmentation may last more than 1 year. Georgiev2 estimates that 1% of his patients, and Duffy6 that up to 10% of his patients, have pigmentation lasting more than 1 year. Izzo1 reports a 2% incidence after 1 year. Our experience parallels that of Georgiev.
Persistent telangiectasia may be caused by factors other than sclerotherapy itself. In certain patients, pigmentation may be present over superficial varicosities and telangiectasias before sclerotherapy is performed.2,20 Hyperpigmentation as a result of ‘physiologic’ diapedesis of RBCs through fragile vessels is common in patients with venous stasis, or over varicose veins. Therefore, preoperative documentation, including photographs, may be beneficial during follow-up patient visits.
Prevention and minimization
Thibault and Wlodarczyk62 recommend that patients avoid taking all iron supplements during the course of treatment and for 1 month after treatment. This presumably decreases serum ferritin levels. Alternatively, patients’ serum ferritin levels may be assessed before sclerotherapy to determine if iron chelation therapy is warranted. Obviously, this latter recommendation awaits further study. We think it is prudent to ask our patients who have developed pigmentation if they are taking iron supplementation, and, if so, discontinue it before future treatments.
Preventing formation of postsclerotherapy-related ecchymoses would theoretically prevent postinflammatory hyperpigmentation through avoidance of dermal hemosiderin deposition. Although Arnica montana is routinely used by many surgeons to prevent perioperative bruising, the efficacy of this homeopathic product has not been scientifically proven. A recent randomized, prospective, double-blind study evaluated the perioperative use of A. montana (SinEcch) in 29 patients undergoing face-lifts.94 Interestingly, the amount, duration, and degree of bruising did not differ between those treated with 10 days of A. montana and those in the placebo group. Although further procedure-specific studies would be necessary, it is likely that this lack of efficacy would be similar if A. montana was used in conjunction with sclerotherapy.
While laser treatment of telangiectatic leg veins is said not to be associated with pigmentation, we have documented numerous patients treated with a variety of lasers who developed prolonged pigmentation (Fig. 8.9).
Treatment
Treatment of pigmentation, once it occurs, is often unsuccessful unless one has access to a Q-switched laser. Because this pigmentation is caused primarily by hemosiderin deposition and not melanin incontinence, bleaching agents that affect melanocytic function are usually ineffective. Exfoliants (trichloroacetic acid) may hasten the apparent resolution of this pigmentation by decreasing the overlying cutaneous pigmentation or promoting the exfoliation of hemosiderin, but they carry a risk of scarring, permanent hypopigmentation, and postinflammatory hyperpigmentation. However, some physicians have reported apparent success with this therapeutic method.1,95 The combination of 20% trichloroacetic acid with retinoic acid and hydroxyquinoline has also been reported to totally fade pigmentation in 76% of patients whose pigmentation persisted from 6 months to 5 years.1 Pigmentation decreased in the remaining patients. Treatments were given every 7 to 10 days from 4 to 12 weeks.
Chatard20 has found that using light cryotherapy to exfoliate the epidermis and ‘evict the pigment’ is helpful. We have not found cryotherapy useful in our practice.
A seemingly logical form of treatment would be chelation of the subcutaneous iron deposition. Myers96 reported the use of a 150 mg/ mL ointment of disodium ethylenediamine tetraacetic acid (EDTA) in the treatment of 10 patients with pigmentation after sclerotherapy or vein stripping or with pigmentation in chronic postphlebitic legs. He reported a consistent reduction in the shade of the pigmentation in every patient treated. Unfortunately, this was an uncontrolled study, and there have been no further reports of this form of treatment since its presentation in 1965. In our experience, intradermal injections of deferoxamine in an attempt to cause chelation of the hemosiderin appear to be somewhat effective but are painful and expensive. The timing of injections and the concentration and quantity of deferoxamine injected have not been systematically studied.
The topical iron chelator 2-furildioxime (FDO) has been found to provide a level of photoprotection and theoretically may also be useful to treat cutaneous hemosiderin pigmentation.97 However, at the time of that research proposal, Proctor and Gamble had no interest in providing this agent for clinical testing of postsclerotherapy pigmentation (personal correspondence, Oct 1994).
Graduated elastic compression with coadministration of the anabolic steroid stanozolol decreases pigmentation in patients with lipodermatosclerosis who also have varicose veins.98 Skin pigmentation did not change when patients used graduated compression stockings alone. Stanozolol may exert its effect through reduction in perivascular fibrin from fibrinolytic enhancement. In addition, compression alone improves lipodermatosclerotic skin changes, including hyperpigmentation (Partsch H, personal communication, 1992). Although it seems reasonable to promote the wearing of graduated support stockings after treatment, further studies are needed before recommending systemic stanozolol therapy.
A study on the use of 20- to 30-mmHg compression stockings after sclerotherapy treatment of telangiectasia and reticular veins 0.4 to 3 mm in diameter found a decreased incidence of pigmentation when compression was used. Compression for 3 days resulted in a 20% decrease in pigmentation; compression for 1 week produced a 60% decrease in pigmentation compared with no compression; and compression for 3 weeks resulted in limited pigmentation in only 2 of 10 patients.99 This follows the logic of compression reducing vessel lumen size, resulting coagula, and hydrostatic pressure. However, Guex found an even lower incidence of residual pigmentation in a prospective study on reticular and spider veins without compression.100
Finally, laser treatment has been efficacious in 45%24 to 69%101 of patients with pigmentation of 12 or 6 months’ duration, respectively. Hemosiderin has an absorption spectrum that peaks at 410 to 415 nm, followed by a gradually sloping curve throughout the visible spectrum (Fig. 8.10)102,103 The copper vapor laser (CVL) at 511 nm in a continuous airbrush technique and the flashlamp-excited pulsed dye laser (PDL) at 510 nm should interact relatively specifically with the hemosiderin absorption spectrum. Competition from oxygenated hemoglobin (peak absorption at 577 nm) should be low, but interaction with epidermal melanin, which has a higher absorption rate at these wavelengths, may be significant. These lasers are thought to result in physical fragmentation of pigment granules, which are later removed by phagocytosis. However, penetration of laser energy at 510 and 511 nm is limited to 1 mm below the granular layer. Since hemosiderin may occur up to 2.8 mm below the granular layer, nonthermal effects may result in clinical resolution. An inflammatory reaction from thermal or photoacoustic effects may stimulate hemosiderin absorption. Although CVL-treated pigmentation responded better than that treated with PDL therapy, thermal relaxation times used by the latter laser system should be more selective.
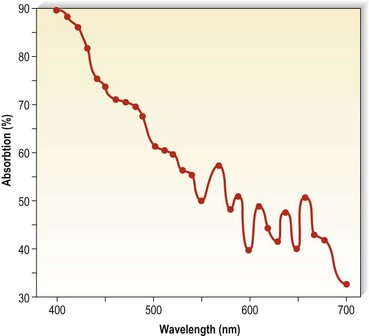
Figure 8.10 Absorption spectra for hemosiderin (freshly frozen, average of two determinations).
(From Wells CI, Wolken JJ: Nature 193:977, 1962.)
The Q-switched ruby laser (694 nm) is also effective in removing recalcitrant pigmentation. Hemosiderin has a peak at 694 nm, and the Q-switching impulse at 20 to 30 nanoseconds is effective in removing tattoo granules. In addition, 694 nm is not absorbed to a significant extent by epidermal melanin or hemoglobin and thus has a relative specificity for dermal hemosiderin. An older ruby laser (Laseaway, England) was been found to effectively remove or minimize pigmentation in a number of patients (Fig. 8.11)104 In a study of eight patients with pigmentation still present 1 to 2 years after sclerotherapy, 92% of the lesions lightened with treatment; 58% of lesions demonstrated significant (75–100%) resolution after one to three (average 1.7) treatments. The Laseaway ruby laser was used with a 4-mm beam size and a fluence range of 5.6 to 10.5 J/cm2. Weiss and Weiss105 could achieve 90% resolution of pigmentation that had been present for an average duration of 18 months with 3.2 treatments using a Q-switched ruby laser as well (Ruby Star, Aesclepion-Meditec AG, Jena, Germany) at 5 to 10 J/cm2 with a 2-nsecond pulse and a 4- to 5-mm diameter spot. We now use a Q-switched ruby laser (Sinon) from WaveLight Laser Technologies (Erlangen, Germany) at 4 to 7 J/cm2 with a 20-nsecond pulse and a 4- to 5-mm diameter spot size. Treatments are performed every 4 weeks until resolution. Care is taken to use the minimal fluence required to produce a whitening of the skin without causing bleeding. Our patients require one to two treatments for complete resolution.
Weiss106 has reported successful clearing with the use of intense pulsed light (IPL; Photoderm PL, Lumenis, Santa Clara, Calif.) in 10 patients with pigmentation persisting after 1 to 2 months. He used the IPL at 30 to 40 J/cm2 given in a single 4-msec pulse with a 590-nm cut-off filter. Significant lightening occurred in 6 of the 10 patients.
The most recent Q-switched laser used to treat postsclerotherapy pigmentation was a novel 532/1064-nm Nd:YAG laser (Palomar Med Tech, Burlington, Mass.). Ten patients who had had persistent pigmentation for an average of 18 months were treated at 2-nsecond pulse with 75% 1064 nm and 25% 532 nm simultaneously with a 6-mm diameter spot at 2 J/cm2. There was a 75% resolution in an average of 2.8 treatments.107
Temporary swelling
Etiologic factors
Multiple factors are responsible for swelling of a treated area. These factors include changes in the pressure differential between the intravascular and perivascular space and changes in endothelial permeability. Edema is most common after treatment of varicose veins or telangiectasias below the ankle, because of the increase in gravitational intravascular pressure in this area and the relative scarcity of perivascular fascia at the ankle. Riddock108 speculates that edema is caused by an unduly prolonged reflex spasm spreading to some of the subfascial (deep) veins.
The extent of edema appears to be related to the strength of the sclerosing solution. This result apparently correlates with the degree of perivascular inflammation produced by the sclerosing solution. The byproducts of inflammation, including release of histamine and various mediators, increase endothelial permeability. In addition to the degree of inflammation induced by sclerotherapy itself, the innate sensitivity of a patient’s perivascular mast cells (possibly related to their atopic or asthma history), concomitant medications that may promote or inhibit mast cell degranulation (e.g. corticosteroids, antihistamines, nonsteroidal anti-inflammatory agents), and previous exposure sensitivity to the sclerosing agent may all contribute to edema. Duffy8 and Goldman13 estimate that the occurrence of pedal edema ranges from 2% to 5%.
Prevention and treatment
Two techniques may decrease temporary swelling. First, perivascular inflammation must be limited, as previously described (see the sections ‘Recommended sclerosing solution amounts and concentrations’ and ‘Postsclerotherapy compression’ in Chapter 9). Ankle edema occurs much less frequently if the quantity of sclerosing solution is limited to 1 mL per ankle.
One method that we have found helpful is topical application of a strong-potency corticosteroid cream, lotion, or gel. Methylprednisolone acts both to stabilize mast cell membranes, preventing histamine release, and to exert part of its anti-inflammatory action directly on the endothelial cell, rendering it less responsive to various mediators.109 Ruscus extract inhibits macromolecular permeability, increasing the effect of histamine in the hamster cheek pouch model.110 This effect is due to stabilization of endothelial pore size. Beta-receptor agonists such as terbutaline and theophylline counteract histamine-induced venular permeability. This effect also occurs with verapamil and glucocorticoids.111
A second method for limiting the degree of pedal or ankle edema is application of a graduated pressure stocking routinely after injections in this area.112 One study compared the use of postsclerotherapy graduated compression for 3 days to 3 weeks and reported that none of 10 patients complained of edema when stockings were worn for 3 weeks; 40% of patients who did not wear post-treatment compression stockings had edema, with 30% having edema if stockings were worn for only 3 days and 20% complaining of ankle/pedal edema if stockings were worn for 1 week.99
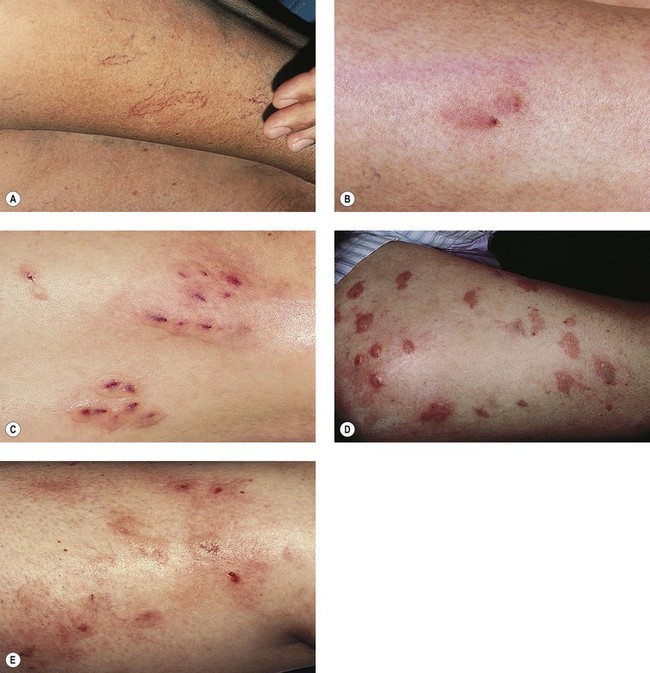
Telangiectatic matting
The new appearance of previously unnoticed, fine red telangiectasias occurs in a number of patients after either sclerotherapy or surgical ligation of varicose veins and leg telangiectasias (Figs 8.12 and 8.13). This occurrence has been termed flares by Arenander and Lindhagen,113 distal angioplasia by Terezakis,114 blushing by many, and telangiectatic matting (TM) by Duffy.6 The reported incidence varies from 5%13 to 75%.14,43,113 Two retrospective analyses of 2120 and 7200 patients with leg telangiectasia each reported a 16% incidence.15,115 This incidence has been confirmed in a random sample of 113 female sclerotherapy patients.42
Although the average severity was considered minimal, telangiectatic matting was noted in approximately 70% of our patients treated with foam versus 84% of those treated with liquid STS sclerotherapy.17 Of note, however, all evidence of new vessel formation had resolved completely within several months (on average within 3–6 months). Reasons for the development of TM are multiple. Recovery from an ischemic injury such as closing blood vessels with sclerotherapy may produce a hypoxia-induced neovascularization. In addition, injury to endothelial cells may stimulate the release of a variety of growth factors.
These responses are probably a fundamental feedback response, acting to satisfy tissue needs for oxygenation. For example, this response is commonly seen in myocardial collateralization. Circulating endothelial progenitor cells, elevated with estrogen therapy, may also lead to neoangiogenesis.116 Given these protective factors, it is curious that the incidence of TM after sclerotherapy is not higher; therefore, other innate factors must predispose to the development of TM.
The incidence of TM increased with increasing patient age in one report,113 but this correlation was not observed in another report.115 Although most authors do not comment on a gender predisposition,8 we have seen the development of TM in only one male patient with leg telangiectasia. Because fewer men than women seek treatment for leg telangiectasia, an accurate appraisal of the gender incidence of TM cannot be stated.
TM may appear anywhere on the leg but we have never seen it occur on the face, hand, or chest after sclerotherapy treatment. One detailed study found that most TM occurred on the medial ankle and the medial and lateral calves.113 However, TM also has been reported to occur more frequently on the thighs.117 Duffy has reported that in 80% of his patients TM developed within 10 inches (25 cm) above the knees (personal communication, Oct 1994). Our experience is similar to Duffy’s. Duffy postulates that relative ischemia occurs in this area from tissue hypoxia that results from the thighs and knees pressing on each other during sleep when persons lie on their side. Hypoxia has been found both in the retina and around compressive tumors to promote vascular endothelial growth.118–120 Although a 14.5% incidence of TM has been reported when treating hand veins,59 we have not seen this in hundreds of hand veins treated by sclerotherapy.
Etiologic factors
Postsclerosis TM was first described in the 1960s by Ouvry and Davy.121 They observed that the incidence of matting was proportional to the degree of inflammation and thrombus formation. The etiology of TM is probably related either to angiogenesis8,122 and/or to a dilation of existing subclinical blood vessels by the promotion of collateral flow through arteriovenous anastomoses.122,123
Probable risk factors for the development of TM in patients with leg telangiectasia include obesity, use of estrogen-containing hormones, pregnancy, and a family history of telangiectatic veins. Excessive standing does not appear to influence the incidence of TM.115 Excessive postsclerotherapy inflammation also may predispose toward development of TM.
After sclerotherapy, the development of TM occurs rapidly; often patients report the development over a few days at 3 to 6 weeks after treatment. Normally, the more than one trillion endothelial cells that line blood vessels have a turnover time of more than 1000 days.124 However, under appropriate conditions, new vessels can develop in 2 to 3 days. Observations of mammalian systems have demonstrated the development of a vein from a capillary, an artery from a vein, a vein from an artery, or from either back to a capillary.125,126 In coronary vessels, the number of arterioles and capillaries increases within 1 week after injury.127
Angiogenesis
Angiogenesis is a complex process in which capillary blood vessels grow in an ordered sequence of events. Angiogenic factors act either directly on the endothelium to stimulate locomotion and mitosis or indirectly by mobilization of host helper cells (mast cells and macrophages) with release of endothelial growth factors (see below). When a new capillary sprout grows from the side of a venule, endothelial cells degrade basement membrane, migrate toward an angiogenic source, proliferate, form a lumen, join the tips of two sprouts to generate a capillary loop, and manufacture new basement membrane.128 Obstruction of outflow from a vessel (which is the end result of successful sclerotherapy) is one of the most important factors contributing to angiogenesis.129 Initiation of angiogenesis also follows disruption of endothelial continuity or intercellular contact. This contact results in endothelial cell sprouting and migration.130
Hypoxia with a decrease in the partial pressure of cellular oxygen also is a potent stimulator of neovascularization. With ischemia, one of the first genes upregulated is the gene encoding hypoxia-inducible factor-1 (HIF-1).131 This factor activates genes involved in angiogenesis, glycolysis, modulation of vascular tone, and erythropoiesis.131–133
In addition, endothelial damage leads to the release of heparin and other mast cell factors that both promote the dilation of existing blood vessels and stimulate angiogenesis.134–137 Finally, neovascularization may be promoted by numerous other angiogenic factors, including, but not limited to, heparin-binding fibroblast growth factor (FGF),2,138,139 tumor necrosis factor (TNF),135,140,141 platelet-derived endothelial mitogen,142 endothelial cell growth factor (ECGF),143 and other macrophage-derived growth factors.144,145 These factors and many others are released from perivascular mast cells.146 FGF is released at cell death and is essential for the stimulation of angiogenesis and wound repair.147,148 Thus, sclerotherapy, through endothelial damage, promotes the release of both endothelial angiogenic factors and perivascular mast cell angiogenic factors that provide multiple mechanisms for the formation of new blood vessels. Indeed, it is remarkable that postsclerosis TM does not occur more frequently.
Sclerotherapy-induced perivascular inflammation also may promote TM.26 Inflammation may be considered a hypermetabolic state, with new vessel growth occurring as a result of increased metabolic demand.149 In addition, mast cells are found in increased numbers in inflammatory states, such as allergic contact dermatitis or delayed hypersensitivity reactions.150 Since mast cell heparin is one factor responsible for capillary endothelial cell migration,124 the degree of inflammation should be limited as much as possible to decrease angiogenic stimuli. This is achieved by choosing an appropriate solution concentration for each type of vessel treated, limiting the quantity of solution to the amount that will not produce excessive endothelial damage, and limiting the size of postsclerotherapy thrombosis. This was confirmed by Weiss and Weiss,42 who found that in a random sample of 113 sclerotherapy patients, TM developed in 10 with injection of POL 1% into vessels less than 1 mm in diameter. When POL 0.5% was used for subsequent treatments in these patients, further areas of TM did not develop. The use of low concentrations of POL was found in a multicenter report of 16,804 legs to have an incidence of TM of 0.04%.151 However, it is unclear how closely patients in this large prospective clinical trial were followed. A comparison of POL 1% with HS 20% in treating leg telangiectasia showed that the incidence of TM was higher with the more caustic POL (36%) than with HS (31%) (Fig. 8.14).44
Another group of investigators reported TM in 12% of patients treated with Sclerodine 0.25%, in 17% treated with Sclerodex, and in 15% treated with 0.25% POL.12 These agents should all be comparatively similar in their sclerosing power despite their different mechanism of action on endothelial cells. Interestingly, although their effectiveness in eliminating telangiectasia varied from 73% for POL to 44% for Sclerodine and Sclerodex, the incidence of TM was relatively similar.
A study comparing different times of postsclerotherapy compression in treating leg telangiectasia also demonstrated a decrease in TM when compression was maintained for 1 to 3 weeks (5%) versus 3 days (30%) or no compression (40%).152 This is most likely a reflection of a decrease in intravascular thrombosis with prolonged graduated compression, which results in a decreased phlebitic effect with decreased inflammation.
As noted previously, assuming a role for the perivascular mast cell in the etiology of TM is intriguing. With aging, cutaneous mast cells decrease by 50%, associated with a 35% decrease in subepidermal venules.60 Thus, if TM develops predominantly from mast cell factors, its incidence should be decreased in the elderly; however, this has not been observed in two studies.113,115 Cutaneous mast cells usually occur perivascularly, with a distribution ranging from 7000/mm to 20,000/mm.7,8,153–156 This represents 0.2% to 0.7% of normal skin. In telangiectatic macules associated with mastocytosis, mast cells account for 3.5% (±1.8 SEM) of cells, whereas telangiectasia not associated with mastocytosis has a mast cell volume of 0.4% (±0.1 SEM).157 An analysis of mast cell content in TM lesions is needed.
Estrogen may play a role in the development of TM. It appears that the incidence of persistent TM may be increased in patients taking systemic estrogen preparations.8,42,115 Weiss and Weiss42 found a relative risk of 3.17 (P < 0.003) for development of TM while patients were receiving exogenous estrogen. Sadick also found an increased incidence of TM (10% vs 4%) in patients on oral contraceptive agents or hormone replacement therapy.158 The mechanism for promotion of TM by estrogen is speculative but may be the result of its effect on modulating mast cell responses.159
Estrogen receptors have been found in a number of tumors, including angioma of the nose, soft tissue sarcoma, breast carcinoma, endometrial carcinoma, and unilateral nevoid telangiectasia syndrome. Estrogens also play a role in the development of vascular tissues. In vitro, estrogen and estradiol have promoted endothelial cell migration and proliferation.160,161 Spider angiomas develop during pregnancy and resolve after delivery.162,163 Spider nevi also occur in patients with hepatic cirrhosis associated with elevated serum estradiol levels.164 In addition, Davis and Duffy105 have reported on the virtual disappearance of leg telangiectasia and TM in a 51-year-old woman with estrogen-receptor-positive breast carcinoma after initiation of antiestrogen therapy with tamoxifen citrate (nolvadex). This may be due to the inhibition of angiogenesis by tamoxifen.165,166 However, although it seems logical that estrogen plays a role in the development of TM, estrogen receptors could not be demonstrated in biopsy specimens from leg telangiectasia.167 An evaluation of estrogen receptors in 10 patients with TM lesions did not demonstrate estrogen/progesterone receptors as assayed by ERICA/PRICA technique.168 The limiting factor of this study as stated by the authors was the small size of the study group, as well as the possibility that the immunocytochemical and radioligand assays may not have been sensitive enough to document a small number of estrogen or progesterone receptors. In addition, a selective estrogen-receptor modulator that is specific for blood vessels has yet to be identified.169 Further, circulating endothelial progenitor cells are also elevated with estrogen therapy and may lead to neoangiogenesis.116 Since estrogen receptors have been implicated in the promotion of angiogenesis,170 it may be prudent, albeit premature, to withhold estrogen therapy during sclerotherapy treatment until double-blind controlled studies have been performed.
Prevention and treatment
Regardless of the cause of TM, since patients seek treatment to eliminate leg telangiectasia, it is disconcerting for the sclerotherapist to produce new areas of telangiectasia. Unfortunately, even in the most expert hands, TM occurs in a significant percentage of patients. Fortunately, TM usually resolves spontaneously over 3 to 12 months.8,42 It has been estimated that 10% to 20% of patients will have persistent TM.115,171 Our experience is that less than 1% of patients will have TM persisting for 1 year.
Various vascular-specific lasers and IPL sources may be useful in treating these vessels.172–176 In our practice, at least 75% of patients with persistent TM partially or completely improve after laser or IPL treatment. Interestingly, individual TM lesions may respond better to one laser or IPL than another. Reasons for the variable response are speculative. The 532-nm long-pulse Nd:YAG laser set at the highest fluence and pulse durations available has been found to be most effective on the most recalcitrant lesions. However, persistent and, rarely, permanent hypopigmentation may occur. The use of the PDL may also be effective but result in long-term hyperpigmentation. Glaich et al recently reported successful treatment of postsclerotherapy matted telangiectasias using fractional photothermolysis.177 Specifically, marked improvement was noted in these telangiectasias, which had been present for longer than a year, after five successive treatments with a 1550 nm fractional photothermolysis laser at 4 week intervals. The reason for this reported success is questioned as in this study resolution required 6 months, which is the time course of spontaneous resolution of TM. In addition, this was a single case report. We therefore can not recommend fractional photothermolysis as effective at this time. (A complete discussion of laser treatment is found in Chapter 13.) Unfortunately, even with the aformentioned therapeutic approaches, rare TM may remain resistant to treatment, possibly because in certain cases TM may have a feeding arteriolar network that prevents persistent vessel elimination.
In patients who demonstrate a propensity for the development of TM, additives to the sclerosing solutions or topical agents may minimize this complication. Protamine blocks the ability of mast cells and heparin to stimulate migration of capillary endothelial cells.178 Protamine also prevents the neovascularization induced by an inflammatory agent when it is applied locally or given systemically.179 It has no effect on established capillaries that are not proliferating.180 In addition, beta-cyclodextrin tetradecasulfate administered with cortexolone is a potent inhibitor of angiogenesis.181
Systemic treatment before or during sclerotherapy also may be helpful in limiting TM. Through suppression of TNF synthesis, pentoxifylline (Trental) may minimize angiogenesis.182,183 Inhibition of mast cell mediators with the cell wall-stabilizing medication ketotifen also may help prevent TM, edema, and localized urticaria. Ketotifen, a benzocycloheptathiophene derivative, has H1 antihistaminic properties in addition to decreasing mast cell mediator release.184,185 Ketotifen may also exert its effect by depleting mediators in cutaneous mast cells and so requires multiple doses over a few days for maximal effect.186 Its clinical beneficial effect in patients with chronic idiopathic urticaria, cutaneous mastocytosis, and urticaria pigmentosa has been established.186–190
Pain
Prevention
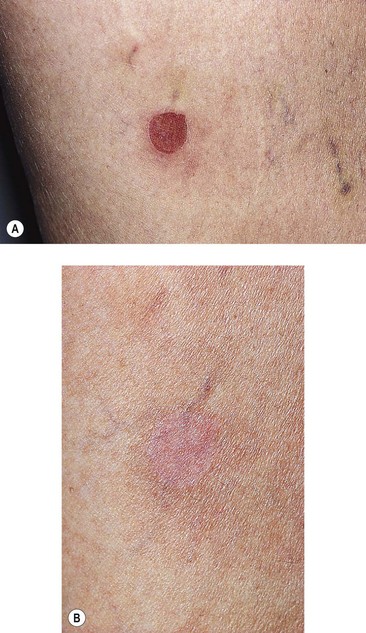
Since most patients who seek treatment of leg telangiectasia require multiple treatment sessions, each consisting of numerous injections, an attempt should be made to minimize the unpleasantness of the procedure. Certain areas are slightly more painful, especially the ankles, feet, upper medial thighs, and medial knees. The authors have not found it necessary to utilize topical anesthetic creams and indeed a commonly used anesthetic cream, EMLA (eutectic mixture of lidocaine and prilocaine) has been found to produce vessel contraction, making it more difficult to perform treatment (Fig. 8.15). Two variables that can be adjusted to minimize pain are the type and size of the needle and the type of sclerosing solution.
Technique
With sclerosing solutions that are inherently painful to inject (e.g. hypertonic solutions), slow infusion can minimize pain.6,8 Slow injection produces a slower distention of tissue and may minimize endothelial cell separation, which may decrease perivascular nerve stimulation.
Type of Sclerosing Solution
Hypertonic solutions are notorious for causing pain on injection. The cramping pain that may develop after correct IV injection usually occurs a few minutes after injection. Weiss and Weiss6 reported that 72% of their patients injected with HS 23.4% felt pain that lasted less than 5 minutes; 4.5% of patients had pain that lasted more than 5 minutes. This pain probably occurs at the time the hypertonic solution reaches the nerve fibers of the adventitia, either through the wall of the vein or through the capillaries. Subsequently, because of stimulation of sympathetic perivenous nerve fibers, an active contraction of the muscle occurs that may also produce a cramping pain.191 In addition, vascular spasm caused by direct effects of the hypertonic solution itself may occur.
Hypertonic solutions also produce muscle cramping after injection. With the injection of 5 to 10 mL of heparsal (HS 20% plus heparin, 10 U/mL) per injection site into varicose veins, Chou et al192 noted that 16% of 310 patients could not tolerate the pain associated with the procedure. Duffy8 estimated that 82% of his patients treated with HS reported moderate cramping or aching. This can be limited somewhat by keeping the volumes injected to 0.1 mL or less per injection site and by massaging the area immediately after injection.
Adding lidocaine to the sclerosing solution may lessen muscle cramping and allow placement of additional injections into the same area with less pain.8,9 A comparison of HS 23.4% with HS 19% diluted from HS 23.4% with a 2% lidocaine solution demonstrated a significant decrease in pain with the lidocaine solution.14 In the HS 23.4% group, 61.9% of patients rated their pain as none to mild, whereas in the group treated with the HS 19%/lidocaine solution, 90.5% of patients reported none to mild pain. There was no difference in efficacy between the two solutions. However, McCoy et al44 found that even with the addition of 2% lidocaine to HS, patients reported significantly more pain with injection as compared to POL 1%.
Chromated, or plain, glycerin solutions are also painful during injection and may produce mild muscle cramping if more than 1 mL of solution is injected into a single vein.47 We have found that the addition of lidocaine 1% to the glycerin solution minimizes pain on injection similar to its effect with HS. Two relatively painless solutions are POL and STS. The advantage of STS is that it is painful only when it is injected into perivascular tissues, thereby providing a noticeable check on inadvertent perivascular injection. Polidocanol is painless with both intradermal and IV injection. Therefore, one does not have the additional sign of pain to ensure accurate placement of the sclerosing solution. In a double-blind comparison of STS, HS, and POL, patients preferred injection with POL.193 Although relatively painless to inject, STS sometimes produce a dull ache a few minutes after injection. This ache resolves in a few minutes and is most likely related to damaged endothelial cells, which release a variety of factors promoting perivascular edema and inflammation; there is no post-treatment aching with POL.
Despite optimal technique and mild sclerosing agents, post-treatment soreness for 1 or 2 weeks after injection occurs in 20% of patients.8 With the use of nonosmotic sclerosing solutions and the use of graduated compression stockings after treatment, we have not seen soreness in most patients after treatment. If patients do complain of soreness, the cause usually is secondary to thrombosed or inflamed vessels or even a poorly fitted graduated compression stocking.
Localized urticaria
Localized urticaria occurs after injection of any sclerosing solution (Fig. 8.16). It is usually transient (lasting approximately 30 minutes) and probably is the result of endothelial irritation with release of perivascular mast cell histamine. Localized urticaria is not an allergic response, since it occurs even after injection of unadulterated HS 23.4%. It is an example of physical urticaria and is frequent in patients demonstrating dermographism.
Urticaria may occur as the earliest manifestation of perivascular inflammation, through release of endothelial- or platelet-derived factors that lead to perivascular mast cell degranulation194 (see previous discussion in sections on pigmentation and edema).
Approximately 40% of patients studied by Norris et al43 described temporary itching after injections with POL, regardless of drug dosage. Duffy8 reported an almost 100% occurrence of urticaria with injection of either POL or HS–heparin–lidocaine solutions. The urticaria is usually more intense when more concentrated solutions are used.8,13 Urticaria also may be more intense with repeat injection sessions, especially when POL or STS is used.
Treatment
In our experience, localized urticaria and itching can be diminished by applying topical steroids immediately after injection and by limiting the injection quantity per injection site. This is particularly helpful in patients who will wear a graduated support stocking after treatment. High-potency topical corticosteroids, such as clobetasol propionate, have been shown to rapidly decrease histamine-induced pruritus.195 Therefore we recommend application of a non-greasy, fast-absorbing, high-potency corticosteroid to all treated areas after sclerotherapy. A secondary effect of the topical corticosteroid is vasoconstriction, which also may help with vessel resolution, reduction of TM and minimize post-treatment thrombosis with its sequelae.
Tape compression blister
Tape compression blisters (Fig. 8.17) occur when a tape dressing is applied to an area of tissue movement or to thin, elderly skin. The blister usually appears as a flaccid fluid-filled sac overlying normal-appearing skin. It usually is not associated with induration or erythema of the adjacent skin. Common sites of occurrence are the posterior calf, medial thigh, and popliteal fossa.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree