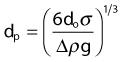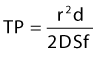CHAPTER 9 Clinical Methods for Sclerotherapy of Varicose Veins
‘We feel from our three and one-half years’ experience that the surgeon who believes that there is nothing more required than a syringe, some solution, and a patient to effect permanent obliteration of varicose veins, still has much to learn.’1
Varicose veins represent tortuous dilations of existing superficial veins. They arise because of multiple factors but are always associated with a relatively elevated venous pressure.2 Therefore, it has been considered for decades that treatment initially consists of cutting off the point of high-pressure inflow to the veins (through either surgical or sclerotherapeutic methods) before treating the varicose veins themselves.3,4
This approach has been recently questioned, since, in certain cases, the importance of the ‘siphon’ effect generated by the underlying varicose reservoir is strong enough to generate a reflux in saphenous trunks. It has been observed that suppression of the varicose network reduces reflux in the saphenous trunks. The development of varicose veins in the reticular network and centripetal progression of the disease, as advocated by Hébrant and Collignon, is a hypothesis which could explain a number of varicose patterns, and an increasing number of specialists are currently applying a more distal and conservative approach, whether surgical or sclerotherapeutic.5
Historical Review of Techniques
Tournay (French) technique
The Tournay procedure encompasses the basic principle of ‘French phlebology’ developed by Tournay et al:6 treating varicose veins ‘from high to low’ (‘de haut en bas’). The rationale for this technique is in first eliminating high-pressure reflux blood flow at the point of occurrence. Treating from proximal to distal sites also eliminates the weight of the column of blood on the sclerosed point. This has the advantage of minimizing thrombosis and the extravasation of red blood cells (RBCs) from a sclerosed vein segment. The French school advocates placement of very few injections at this ‘high (proximal)’ point before treating more distal veins or sections of the same vein at a later date. This same philosophy toward treatment was also reported from the Mayo Clinic in 1941 by Heyerdale and Stalker.7 The principle of eliminating reflux from the saphenofemoral junction (SFJ) was espoused even earlier by Moszkowicz8 in Germany in 1927 and by de Takats and Quint9 in the United States in 1930. Thus the ‘French technique’ is multicultural in its origin.
In addition to developing a treatment regimen with the tenet just mentioned in mind, it is critical to obliterate the SFJ accurately because its location and anatomy are so variable (see Chapter 1). To determine the origin of high pressure in the varicose vein, a noninvasive diagnostic evaluation of the patient should be performed first (see Chapter 5). The handheld Doppler device helps detect points of reflux from the deep to the superficial veins, through either incompetent saphenofemoral or saphenopopliteal junctions or perforating veins. In certain circumstances, additional testing is required. If any points of reflux are detected, they should be treated first. After the high-pressure flow has been eliminated, treatment should proceed first with injection of the largest varicose veins, then injection of the reticular feeding veins, and finally treatment of the remaining ‘spider’ veins.
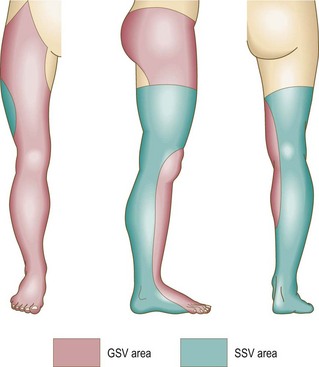
In defense of the logic of the French technique, de Groot4 pointed out that any vein in the leg belongs to either the greater saphenous system or the lesser saphenous system (Fig. 9.1). This concept implies that a vein within the defined area of the great saphenous vein (GSV) eventually drains into the GSV. Therefore, reflux in that vein is derived from reflux at the SFJ. This concept directs treatment toward the reflux point. Unfortunately, clinical examination of the location of a vein does not always correctly determine the point of reflux. Therefore the clinical examination must be correlated with a noninvasive examination to establish the optimal order of therapy.
Sigg (Swiss) technique
In contrast to the French technique, the Swiss technique of Sigg10 and the modification by Dodd and Cockett11 advocate total sclerosation of the entire varicose vein, including incompetent perforator veins (IPVs) and the SFJ. This technique has also been adopted by some physicians who modify Fegan’s technique of sclerotherapy of the IPVs (described in the following section). Included in this school are Reid and Rothnie,12 who treated 1358 legs of 974 patients and found that selective injection of perforator veins was ineffective, requiring multiple additional treatments of the entire vein. They evolved a technique of placing multiple injections at intervals along the varicose veins to produce diffuse sclerosis and have reported very favorable results. This latter technique is called the total-vein sclerotherapy technique.
Fegan technique
Fegan et al13 proposed a view opposite to the previously mentioned one, namely that saphenofemoral incompetence could occur as a result of perforator incompetence alone. They reached this conclusion by demonstrating that the calf muscle pump is more powerful than the abdominal or femoral muscles regarding venous flow in the leg. They proposed that sclerotherapy of the IPVs should be performed first, to restore normal function. Fegan’s examination of the SFJ and GSV with phlebography after sclerotherapy of IPVs showed narrowing of the vessel lumen in 9 of 11 patients.14 However, surgical exploration of clinically diagnosed areas of perforator vein incompetence has found at best a 60% incidence of perforator veins being correctly identified clinically.15 In addition, phlebographic examination performed on 112 patients with clinically suspected incompetent perforators showed that the clinical examination correctly identified only 38% of perforator veins below the knee and only 17% of thigh perforators.16 An additional study of 180 limbs with primary varicose veins studied with clinical examination, ascending deep-to-superficial venography, Doppler ultrasound, and ambulatory venous pressure measurements showed that only 40% of these patients had evidence of perforator incompetence. Of them, 30% had no hemodynamic significance to the perforator veins.17 Thus, the rationale for Fegan’s technique is open to dispute.
Despite the lack of accuracy of clinical diagnosis of IPVs, many authors have found Fegan’s technique gives excellent results. Tolins18 reported favorable results using Fegan’s technique; he injected varicose veins in areas of fascial defects with 0.5 mL of sodium tetradecyl sulfate (STS) solution at up to 23 sites per leg. Although this many injections may seem similar to the number with total vein sclerotherapy, three-quarters of the patients had two to five injections. Doran and White19 concluded after 2 years of follow-up that there was no difference between the results from Fegan’s technique and ligation and stripping procedures for varicose veins. Hobbs20 concluded from his comparative study of sclerotherapy with Fegan’s technique versus traditional surgery that sclerotherapy is the best treatment of nontruncal varicose veins and IPVs of the lower leg. Tretbar and Pattisson21 have found in their follow-up examinations of 264 patients treated with Fegan’s technique that treatment failures usually occurred in patients with very large or fat legs with varicosities originating above the knee. They believed this failure was caused by the difficulty of placing injections accurately within the veins of these patients and maintaining adequate compression. Sladen22 analyzed 263 limbs with up to 7 years of follow-up and found that more than 95% of his patients were satisfied with the treatment and said they would have it repeated. He estimated a retreatment rate of approximately 5% per year. His patients averaged 3.6 to 5.25 injections per treatment session, and 46% to 74% required one treatment session only. Sladen, in agreement with Hobbs, found that all patients with saphenofemoral reflux eventually required surgery. Therefore, although varicose veins treated with Fegan’s technique respond well to treatment, the supposition of Fegan’s technique – that sclerosis of the IPVs is of primary importance and may reverse the remaining pathology in the GSV system – may not be correct universally.
Hobbs23 provided evidence that when saphenofemoral and perforator incompetence occur together, both abnormalities should be corrected. Kerner and Schultz-Ehrenburg24,25 studied the functional effects of sclerotherapy with photoplethysmography (PPG) and concluded that the greatest functional improvement occurred with obliteration of the SFJ. Obliteration of the IPVs of the lower legs was of variable importance. Treatment of perforator veins of the upper leg had no functional significance that could be ascertained with PPG.
Treatment of reflux from the saphenofemoral junction
There are at least three schools of thought regarding which type of therapy is appropriate for initial treatment of the junctional points of reflux: surgical ligation (with or without limited stripping) of the SFJ versus sclerotherapy of the SFJ versus sclerotherapy of IPVs alone. Bergan (see Chapter 10), Goldman,26 Hobbs,20,27 and others argue that surgical treatment of the junctions is the most appropriate and successful mode of treatment, but this was before the use of endovenous ablation techniques. Neglen,28 in a comprehensive review, determined that with proper follow-up, including functional testing at 5 years, surgical therapy of the GSV in a patient with incompetence of the SFJ, is significantly better than sclerotherapy.28 Color-flow duplex evaluation, from 3 to 55 months after treatment (mean of 27.5 months) of 89 limbs in 55 patients with an incompetent SFJ treated with the Sigg technique, found that only 6% of veins remained sclerosed despite improvement in symptoms in 50%.29 Butie30 has shown that sclerotherapy of the SFJ is difficult and unreliable with the use of liquid STS 3%, which is the strongest sclerosing agent approved for use by the US Food and Drug Administration (FDA). This has been confirmed even when sclerotherapy was performed at the SFJ under angioscopic guidance (see ‘Endoscopic injection’ section).31 In this case, 12 GSVs were occluded at the SFJ through angioscopically guided sclerotherapy with STS 3% liquid (prior to the use of foamed detergent sclerosants). A total of 2 to 5 mL was injected into the GSV just below the junction, which was occluded by manual pressure. Nine veins were evaluated at follow-up, and all were reopened and incompetent between 1 and 12 months. Although these carefully performed, unbiased studies have been carried out only recently, this opinion is not new. As far back as 1934, Cooper,32 in a series of more than 85,000 injections in more than 3000 patients, documented that the number of sclerosing injections required to produce obliteration of the varicose vein was markedly decreased after ligation of the SFJ.
In addition to its lack of consistent success, sclerotherapy of the SFJ has the inherent risk of damaging the deep venous system and the femoral vein when sclerosing solution is injected in the upper thigh region. This has been demonstrated by radiologic examination showing the rapid flow of contrast media from a varicose GSV into the femoral vein when injections are performed in the upper thigh.33 With incompetence of the SFJ, it is recommended that sclerotherapy be used for treating residual varicosities after surgery.
Despite the information in the preceding paragraph, multiple phlebologists have demonstrated that the junctions can be successfully closed with sclerotherapy alone in 50% to 91% of patients.34–40 A comprehensive illustrated discussion of the technique is presented in other sources.41 The difficulty in properly evaluating these conclusions is due to the natural evolution of varicose veins, which obscures the results of both surgical and sclerotherapy treatment. It is sometimes difficult to distinguish between a recanalized vein and a new vein. Results also depend on the type of follow-up examinations (i.e. clinical or objective with duplex or Doppler ultrasound) of patients. One study with clinical 6-year follow-up demonstrated a nearly 90% success rate with sclerotherapy.34 By whatever method, closure of the SFJ has been shown in pressure studies to prevent retrograde flow in the saphenous and perforator systems.42 This suggests that incompetence of the perforator veins occurs as a result of a primary development of saphenofemoral reflux. Therefore, when the GSV or its tributaries are involved, the SFJ, when incompetent, should be the first area to be treated.
Treating incompetence of the SFJ may differ from treating an incompetent saphenopopliteal junction (SPJ). The small saphenous vein (SSV) has a variable termination (see Chapter 1) that is often difficult to approach surgically, but can be approached with modern endovenous ablation techniques. Ligation alone at the SPJ has a 95% failure rate at 1 year.43 Sclerotherapy is much more effective, perhaps because of the larger extent of destruction of abnormal feeding veins into this region.43
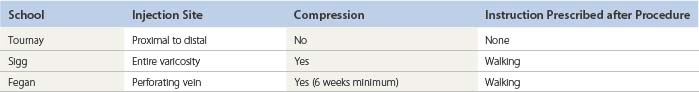
A comparison of three independent observers regarding treatment outcome of varicose vein surgery shows 60% agreement in assessing visual improvement, with 30% agreement between observers when symptomatic response and visual impression are compared.44 It is my opinion that no one ‘school’ is absolute but that the correct order of treatment should be individualized for each patient. Some patients have only perforator incompetence and a normal SFJ; thus, Fegan’s technique would be adequate. Patients with saphenofemoral incompetence require treatment of that junction before treatment is initiated elsewhere. Still other patients have no obvious hemodynamic cause for the origin of their varicose veins, thus directing treatment toward the entire varicosity, as described by Sigg.45 A careful workup of all patients is necessary before therapy is begun. In addition to deciding on the sequence of treatment, the physician must consider the various modifications of the injection procedure that some physicians profess have various benefits. As before, comparative and objective studies of these treatment modifications have not been reported. This chapter discusses sclerotherapy treatment of varicose veins, perforator veins, and the SFJ and reviews the available literature. Variations in treatment are addressed, and illustrative cases are presented. A summary of the three schools of sclerotherapy is found in Table 9.1.
Injection Technique
Patient position
Standing
Although the previous description of the treatment of varicose veins seems simplistic, the actual treatment methods for achieving effective sclerotherapy are numerous. Until the 1950s, sclerotherapy was performed with the patient standing throughout the procedure. The purpose was both to distend the varicose vein, allowing easier needle insertion, and to produce firm thrombosis of the treated vein.46 Multiple disadvantages of varicose thrombosis were realized (see Chapter 8), and various methods were devised to limit postsclerotherapy thrombosis. Additionally, injecting sclerosing solution while the patient is standing forces the injection to occur against the hydrostatic pressure of a large column of blood. This may cause the solution to seep along the needle into the perivenous tissues, possibly leading to a chemical phlebitis or tissue necrosis.47
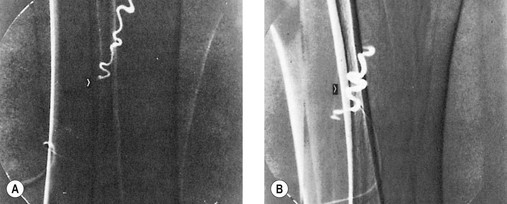
Another disadvantage of the total standing technique is the sclerosing solution may escape through a perforator vein and damage the deep veins, especially if more than 1.5 mL of sclerosing solution is injected in a single site.48 Another radiographic study has shown that an injection of 0.5 mL of contrast media travels rapidly (in 5 seconds) 8 cm distal to the injection site.49 Also, this amount of contrast does not completely fill the vein. An additional radiographic study of the standing position, using metrizamide (Amipaque) 150 as the contrast media (diluted to an isomolarity and specific gravity similar to that of polidocanol (POL)), showed that the injection of 1.5 mL remained in contact with a convoluted varicosity for approximately 10 seconds before flowing rapidly into the deep venous system through a presumed perforator vein (Fig. 9.2, A and B).50 In contrast, when the patient was supine, the contrast media extended proximally along the varicosity and remained relatively undiluted for more than 18 seconds (Fig. 9.2, C and D).50 However, injections in some patients, when using the latter technique, also resulted in the contrast media’s remaining in contact with the varicosity for a longer period with the patient in the standing position (Fig. 9.3). Therefore, multiple variables, including the type of varicosity, its location, the location of associated perforator veins, and the movements of the patient (with associated calf and foot muscle contraction) while standing, may all affect the distribution of the sclerosing solution. For these reasons, the standing technique is not recommended.
Standing and reclining
A modification of the standing technique, the standing and reclining technique, was described in 1926 by Meisen.51 In the standing position, the needle was inserted while the vein was distended. With the needle still in the vein, the patient reclined on a table and the leg was elevated. This produced a relative emptying of blood from the vein. The sclerosing solution was then injected into an ‘empty’ vein that was immediately compressed to prevent or minimize thrombosis.
One of the earliest methods used to limit thrombosis was that of isolating the injected vein segment with pressure placed above and below the needle insertion site after first milking the blood out of the vein.52 One of the first books on sclerotherapy treatment of varicose veins is Varicose Veins and Their Treatment by ‘Empty Vein’ Injection.53 In this book, Ronald Thornhill of London detailed his success with injection of a quinine solution into the elevated leg. He massaged the solution throughout the vein, injecting from distal to proximal. He even sclerosed ‘hair veins’ with a dilute solution. Unfortunately, he did not use compression except when ulcers were present, and his patients had to endure weeks of tender veins until they resolved in approximately 12 months.
Lufkin and McPheeters54 emphasized the importance of empty vein injections in a histologic evaluation of treated veins in 1932. Orbach55 has stressed the significance of compressing the vein to minimize thrombosis since 1943. Thus, these modifications are not new. They result both in improved efficacy and in a decreased incidence of complications when the treatment is directed at the SFJ.19 The improved efficacy may be the result of a longer length of sclerosis of the varicose vein, caused by gravitational flow of the sclerosing solution. However, recent evaluations comparing standing and reclining methods of treating varicose veins below the SFJ have not been performed.
Leg elevation (Fegan)
Another obvious disadvantage of the standing technique is the vasovagal reaction (see Chapter 8). To prevent the sequelae of a vasovagal reaction, Fegan56 recommended that patients sit at the end of the examining table with their legs hanging down while the physician, sitting in front on a low stool, inserts the needle. The leg is then raised while the patient fully reclines for 1 to 2 minutes to empty it of blood. The physician stands to support the raised leg, which is rested on the shoulder or against the chest, and the varicose veins are injected. With this or any technique that moves the patient after the needle is inserted, it is important to ensure that the needle is not displaced from the vein, either by fixing it to the skin with tape (if butterfly catheters or needles attached to syringes are used) or by holding the needle while the leg is raised. Since the varicose veins will collapse when the leg is raised, blood withdrawal must be checked as soon as possible after the leg is raised to ensure that the needle has not slipped out of the vein. The lack of spontaneous pulsatile flow from the needle without an attached syringe is proof of nonarterial placement of the needle.
To ensure that the sclerosing solution acts on the intended vein segment during injection, Fegan56 recommended applying pressure with fingers a few centimeters proximal and distal to the injection point. Finger pressure is maintained for 30 to 60 seconds, and the leg is then bandaged from the toes to the injection site. With this method, injections are made only at the points of fascial defects (which are thought to represent sites of IPVs). Injections proceed from distal to proximal sites, with each vein segment or fascial defect treated.
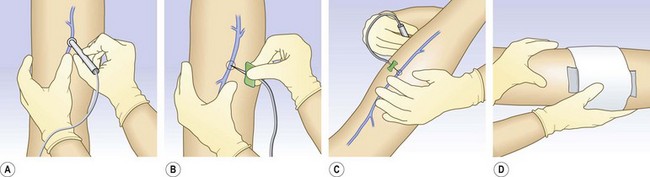
After injection of varicose veins in the elevated leg, compression should be applied immediately to prevent the vein’s filling with blood. This can be performed easily with the use of the following method. Before insertion of the needle, a compression stocking is placed over the foot and heel and allowed to bunch up at the ankle. After the needle is inserted into the varicosity, the leg is raised. Proper placement is then confirmed through blood withdrawal, and the sclerosing solution is injected. A foam pad is placed over the injection site and secured in place with foam or elastic tape. The compression bandage then can be advanced over the injection site and foam pad in a distal to proximal manner (Fig. 9.4). With this technique, radiographic studies after injection of 0.5 mL of contrast medium show that when the leg is raised above the horizontal plane, the contrast medium travels rapidly for 30 cm before reaching the deep venous system.49 The sclerosing solution is diluted and probably inactivated by the time it reaches the deep venous system (see Chapter 7). With this technique it may not be necessary to repeat injections every few centimeters in the varicose vein.
With the leg-raising technique the varicose vein would be presumed empty of blood, but this is not the case. Duplex examination demonstrates that the GSV is not totally emptied of blood, even with an 80-degree incline, although tributaries to the GSV are emptied at 45 degrees.57 In ‘huge’ varicosities, as much as 18 mL of blood can be withdrawn when the leg is raised 45 degrees above the horizontal.58 Therefore, Perchuk58 developed a method for ensuring an ‘empty’ vein. His method consists of inserting the needle into the varicose vein, elevating the leg 45 degrees, and then withdrawing blood through that needle into a syringe until further blood withdrawal is impossible. Then, a syringe with sclerosing solution is attached to the needle and the injection is made, followed by application of local pressure for 5 minutes. The use of compression bandages or stockings after treatment was not mentioned. A two-way stopcock may also be used for this technique. Perchuk, in an evaluation of 84 patients, found that this technique produced excellent results and limited all complications.58 Pigmentation, thrombophlebitis, and recurrence were very rare. Fegan,59 however, disagreed with Perchuk’s conclusions and stated that the vein would empty of blood if raised for a longer time and at a more acute angle.
Two-phase (Sigg) technique
A variation on the method described previously is the two-phase technique of Sigg.10 This variation involves the way the needle is inserted into the vein. Sigg recommended the needle be passed through the vein with the syringe unattached or with a finger placed over the hub and then slowly drawn back until the escape of blood indicates proper placement. The needle is left open and unobstructed while an assistant places a basin beneath it to catch the dripping blood. The leg is then raised above the horizontal plane, and bleeding stops. A syringe is then attached, and blood is withdrawn. After proper placement is confirmed by withdrawal of venous blood and the vein is emptied of blood, the sclerosing solution is injected and the vein compressed, as described above, with a stocking, bandage, or both. Since Sigg uses mainly iodinated iodine solutions, he developed this technique to ensure continual intravascular placement of the sclerosing solution (see Chapters 7 and 8).
Reclining
Another method for injection advocated since the 1920s is that of having the patient remain horizontal throughout the procedure.60 If the varicose veins easily collapse in this position, they can be marked with indelible ink before the procedure, while the patient is standing. A radiologic study has shown a 0.5-mL bolus of contrast medium injected into a varicose tributary of the GSV travels proximally 8 cm and remains within the vessel for approximately 2 minutes before being drawn into the deep venous system.49 The relaxation of the calf muscles permits the injected fluid to stay within the vein, since blood flow in this position is slow. Thus, this position produces the longest lasting and most uniform contact between the injected solution and the vein wall.
To produce a relatively bloodless vein during injection in the horizontal position, some authors advocate rubbing along the vein in both a proximal and distal direction away from the injection site.60 Manual compression is then maintained at the proximal and distal sites along the vein to prevent the vein segment from refilling with blood. After the injection, a compression pad and bandage are immediately applied to the treated vein segment.
Foam sclerotherapy
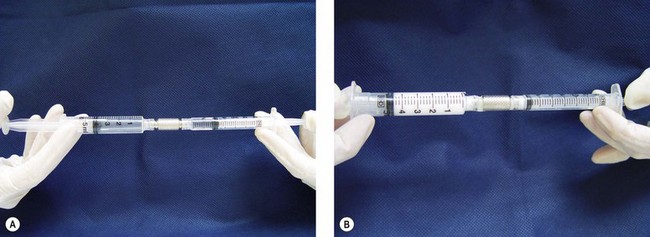
Since the earlier editions of this textbook, the use of foam sclerosants has increased dramatically. Far from the primitive techniques described by so many authors and well detailed in Wollmann’s history of sclerosing foams,61 it is now considered as a completely new treatment for varicose veins.62,63 Shaking the syringe60 or aspirating in a closed glass syringe,64 to quote only two of the well-known historic methods, are now obsolete. Three main options remain: high-speed beating in a carbon dioxide-rich atmosphere (Cabrera’s technique65), specific gas mixture combined with POL and passed through a patented sieve in an aerosol canister (Varisolve, Provensis Ltd., UK), and Tessari’s method, using the transfer between two syringes of sclerosant and room air. The latter technique offers different variations: a three-way stopcock as initially described66 (Fig. 9.5), a two-way female connector like in the DSS technique67 (Fig. 9.6), or an automated foaming device, Turbofoam (KreusslerPharma, France), described in Chapter 7, which has the ability to mix the sclerosant with different types of gases through the use of a three-way connector. The Tessari technique is the most common means for foam creation.68
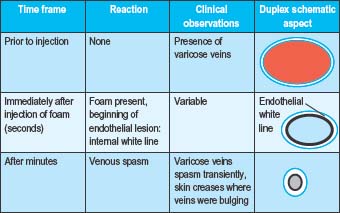
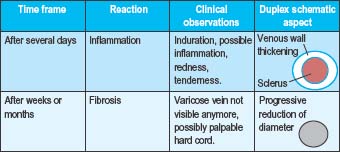
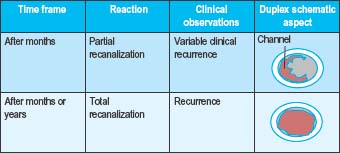
Immediately following the injection of foam sclerosant, the bubbles displace blood and contact with the sclerosant and endothelium begins. Clinically, this is apparent as vasospasm within seconds or minutes, erythema of the feeding telangiectasias, or no immediate visible change (Table 9.2a Pt1). After several days, venous inflammation is present with thickening of the venous wall and presence of a sclerus. This is evidenced clinically by induration, erythema, and tenderness. Weeks to months later, fibrosis results in a progressive decrease in the diameter of the treated vein and clinically by the absence of the varicosity. Occasionally, a fibrous cord may be palpated (Table 9.2b Pt2). At times, complete clearance of the varicose veins does not take place, owing to either partial or total recanalization of the treated vein (Table 9.2c Pt3).
Table 9.2 A-C Pathophysiologic and clinical effects from foam sclerotherapy
Foam sclerotherapy is now regarded as far more efficaceous than liquid sclerotherapy for larger veins due to an increased time of contact between the sclerosant and the vein wall.68 The temporal relationship between foam bubbles and the endovenous wall is related to patient position, injection technique, foam viscosity, and the number of bubbles per milliliter.69 Hamel-Desnos et al67 investigated efficacy rates of a single treatment session of foam or liquid ultrasound guided sclerotherapy of GSVs. The induction of venous spasm was more common following foam sclerotherapy. At 3 weeks follow-up, reflux was absent in 84% of foam sclerotherapy treated veins and 40% of liquid sclerotherapy treated veins. A similar study was performed by Yamaki et al70 to investigate single treatment foam versus liquid sclerotherapy in GSVs and tributary veins. At 12 months, complete occlusion rates were 67.6% and 17.5% in the foam and liquid groups, respectively. Statistically significant increased rates of recurrent varicose veins and recanalization with reflux were identified in the liquid sclerotherapy cohort. A 2009 meta-analysis revealed a 76.8% efficacy of ultrasound-guided foam sclerotherapy (UGFS) as compared to a 39.5% efficacy rate for ultrasound-guided liquid sclerotherapy.71 An additional benefit from foam is its appearance on ultrasound as an intravenous contrast agent. Multiple randomized controlled studies comparing the incidence of adverse events with foam and liquid ultrasound-guided sclerotherapy of the saphenous veins have shown no statistical difference between these techniques.67,71,72
A metanalysis by Van den Bos and colleagues73 compared occlusion rates following endovenous laser ablation (all wavelengths included), radiofrequency (RF) ablation, UGFS, and high ligation with stripping from 64 studies and 12,320 limbs. At 3 years, success rates were 94.5% for laser ablation, 84.2% for RF, 77.4% for UGFS, and 77.8% for high ligation with stripping. After 5 years, the respective treatment success rates were 95.4%, 79.9%, 73.5%, and 75.7%. The authors found efficacy for high ligation and stripping, RF ablation, and UGFS were equal, but endovenous laser ablation therapy (EVLT) was more effective than the other three regimens.
All foams are not the same, as explained by Wollmann.61 Even if prepared with the same agent (foams can be produced only with detergents – namely, STS and POL and, rarely, sodium morrhuate), foams can have different properties depending on the mode of preparation and the gas to air ratio (dilution factor). Foams are wet or dry, accordingly. This difference is thought to have some impact on efficacy. Drier foams tend to break down on passage through a needle while wet foams act more like liquids. Between these extremes, foam is relatively stable and will displace blood from the vein. Liquid sclerosant to gas dilution ratios of 1 : 4 (1 part liquid to 3 parts gas) or 1 : 5 (1 part liquid to 4 parts gas), have been found to produce the most stable foams.74 Currently, most physicians use a foam prepared just prior to injection with a method derived from Tessari and a ratio of solution to air varying from 3 to 5 mL of air to 1 mL of solution (we recommend 4 mL). Some standardization has happened spontaneously.
Foam stability
Foam stability is affected by foam composition, foam volume, and injection technique.75 Composition variables, including the homogeneity of bubble size, viscosity, and temperature, all influence the ‘quality’ and longevity of foam (Table 9.3).76 Heat increases the stability of foam.77
Bubble size is inversely related to the difference in density between a liquid and gas, as represented in the equation below.76
where
Carbon dioxide is 1.5 times denser than room air; therefore, foam bubbles prepared from carbon dioxide are smaller than those of air. Foam bubbles produced via turbulent flow in the Tessari technique and the double syringe system are smaller (less than 100 µm) in size as compared to the larger bubbles produced in the Monfreux technique. This smaller bubble size is associated with an increased surface area of sclerosant, increased displacement of blood inside the vessel, and decreased likelihood of mixing with blood following the initial injection. Therefore, an increased amount of sclerosant can be delivered to the endothelial cells.78,79 Interestingly, the Monfreux method of foam creation has been associated with an increased incidence of side effects. It is speculated that the larger bubbles produced in this method advance more readily in the venous system.78
The majority of phlebologists utilize readily available room air for foam creation in sclerotherapy; however, carbon dioxide is becoming increasingly popular.80 Carbon dioxide foam bubbles quickly disintegrate; this effect is more pronounced as the sclerosant to gas ratio is increased.61 The following equation describes foam stability in vivo:
Carbon dioxide has a much greater diffusibility into blood as compared to nitrogen (the dominant gas in room air), and as a result, the foam-half life is reduced for carbon dioxide. When carbon dioxide is mixed with oxygen for foam creation, the foam-half life is increased due to the decreased diffusibility of this mixture.81
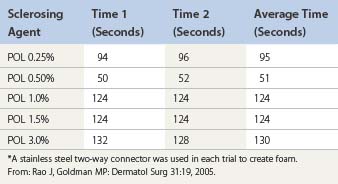
Room air foam half-life varies according to the percent of sclerosant solution. In the study by Rao and Goldman,82 they found a 1% concentration of STS or POL had the maximal foam half-life (90 and 120 seconds, respectively) (Tables 9.4 and 9.5). We recently discovered carbon dioxide foam half-life did not vary according to the concentration of sclerosant solution; however, a mixture of 77% carbon dioxide/23% oxygen did. We also found that foam half-life for room air is over three times longer than carbon dioxide half-life and 1.5 times longer than a combination of 70% carbon dioxide/30% oxygen (see Table 9.3).83
Table 9.4 Times for 0.5 mL of sodium tetradecyl sulfate (STS) to settle from a foam mixture containing 1.0 mL of various STS concentrations*
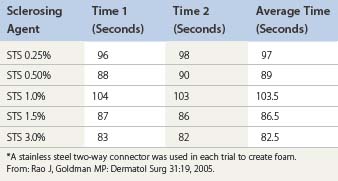
Table 9.5 Times for 0.5 mL of polidocanol (POL) to settle from a foam mixture containing 1.0 mL of various POL concentrations*
Silicone coating is present in syringes and syringe connectors to provide lubrication, but silicone is also an anti-foaming agent. Prior studies by Rao et al and Lai et al, have shown the amount of silicone in syringe connectors does not affect foam stability; however, foam stability varies between syringe manufacturers due to variances in the silicone content of their syringes.82,84 Hill85 investigated the effect of a 5 µm syringe filter on carbon dioxide foam half-life. In the absence of the filter, foam half-life was 22.7 and 30.1 seconds for STS 2% and POL 2%, respectively. Half-life was prolonged to 35.9 and 48.9 seconds, respectively, with the addition of a 5 µm filter. Of note, carbon dioxide foam stability was greater in POL versus STS throughout the study.
Foam has a spontaneous evolution before and after being injected in varicose veins. Before injection, small bubbles tend to group as bigger units (known as LaPlace’s law) and after some time foam is replaced by large bubbles whose effect will be what was observed with Orbach’s air-block. Stability of the foam can be evaluated by several techniques,82 and one should not talk about foam when prepared more than 90 seconds in advance. The preparation of foam does not change the concentration of sclerosing agent. This is completely different from what happens to the foam when injected. If injected into an isolated segment, a plug of foam is created and behaves as in a syringe, remaining in contact with the endothelium long enough to destroy it and induce venospasm. As the foam is forced from the contracting segment of vein, it mixes at the expanding interface with blood. This is responsible for dilution of foam and the scattering of bubbles, and surface sclerosing agent is rapidly deactivated by attachment to plasma protein and cell membranes. This explains why bubbles, when found far from the injection site for example, in the lung, heart, or brain consist of air and do not carry detectable amounts of sclerosing agent or have any sclerosing properties.86
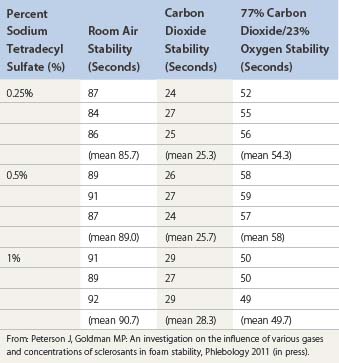
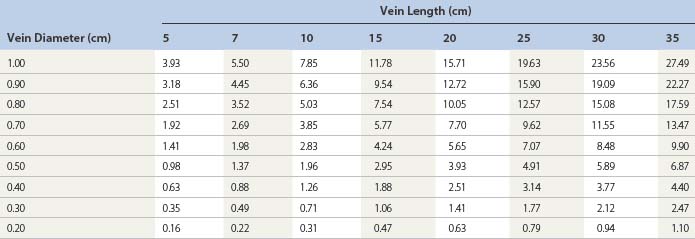
Regarding the use of foam, the trend in Europe is to develop a consensus for volumes and concentrations.80,87 At the 2nd European Consensus Meeting on Foam Sclerotherapy, experts agreed than no more than 10 mL of foam per injection should be injected into a GSV. The range of volume injected into the GSV varied between less than 2 mL up to 10 mL per injection. The maximum recommended volume of foam per leg and per session was felt to be 10 mL. The Monfreux technique of foam creation was no longer recommended for any subtype of vein.80 In France, there appears to be a reduction in concentrations,88 with vessel spasm used as an indicator for the correct amount of foam injected. However, this option is true only for a proximal and direct injection of terminal parts of the saphenous veins. We have previously advocated for a more distal approach62 with placement of an open vein access (‘butterfly’ needle or catheter; Figs 9.7 and 9.8), filling the vein with relatively low volume concentrations, and massaging to the desired area under duplex control (Fig. 9.9). With several years of experience this is no longer deemed necessary. The necessary volume can be estimated from the diameter and the length of vein, as indicated in Table 9.6. In our practice, however, we do not currently use volumes above 10–12 mL in a single vein. It should not be forgotten that although foam is not air and usually occupies the entire vessel lumen, foam floats, so that in large-diameter veins it will principally be in contact with the upper part of the vein, where gravity leads it (Fig. 9.10). The only solution to this problem is a reduction in the diameter, which can be obtained by compression, leg elevation, or induction of venous spasm. Ten to thirty milliliters of normal saline can be injected perivenously, in a technique similar to tumescent anesthesia for endovenous laser or RF ablation, to cause venous compression and decrease recurrence rates.89
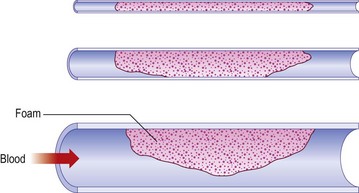
Figure 9.10 Effects of Archimedes’ law on foam contact with venous wall in veins of various diameters.
The most common technique for foam sclerotherapy is via ultrasound guidance81 and using the direct puncture technique.80 While many authors employ short catheters, long catheters with balloon tips have become more prevalent due to a possible increased efficacy. However, long catheters may cause an increased incidence of deep venous thrombosis81 due to a passage through perforators, even if foam is re-aspirated at the end of the procedure. The recommendations from the 2nd European Consensus Meeting on Foam Sclerotherapy is to inject the GSV at the proximal thigh if using the direct puncture technique and distal to the knee if using long catheters. For the SSV, injection should be at the level of the mid calf in order to minimize adverse events including canulization of the popliteal artery.80
Even if every phlebologist using foam sclerotherapy has been impressed by results (see Case Study 13 later in this chapter), evidence-based medicine applies to phlebology too and controlled trials are needed to demonstrate the validity of the technique. Efficacy of foam sclerosants is more substantially documented today than it was when writing the previous editions of this textbook and evidence is now available.67,70,90–92 Cabrera et al93 have followed 500 lower limbs treated with their unique carbon dioxide-foam mixture, with no greater than 81% of the GSV and 96.5% of the superficial branches remaining closed at over 3 years. Results have not been as impressive when room-air-generated foam is used. Fruillini and Cavezzi78 found an 88% success rate when the foam was generated with the glass syringe/Monfreux technique versus 93% when generated with the Tessari technique. Although their follow-up was 20 to 180 days, it is clear that the method for generating foam is important regarding treatment efficacy. Even Tessari reports a 74% efficacy of complete obliteration in a 2-year evaluation of 38 GSVs and SSVs and 194 collateral branches treated.94 In a randomized controlled trial, proprietary pharmaceutical polidocanol microfoam (PPPM; Varisolve) was compared with superficial vein surgery or sclerotherapy in 654 patients with moderate to severe saphenous vein (GSV or SSV) incompetence. The overall success rates were 83.4% for PPPM and 88.1% for control at 3 months, and 78.9% and 80.4%, respectively, at 12 months; at neither time point was the difference significant.95
A 3-year follow-up study in UGFS using either STS or POL for the GSVs, SSVs, and their tributaries revealed a 52.4% success rate. Veins that failed the primary treatment session were re-treated. The 3-year success rate for this group was increased to 76.8%.96 A 5-year 56% efficacy rate was found in a prospective study investigating UGFS prepared from STS of the GSVs. This protocol consisted of weekly foam sclerotherapy treatments until reflux was no longer detected by ultrasound. Additional treatments were performed if recanalization was detected. Currently, this study contains the longest follow-up duration of all published studies on foam sclerotherapy.97 In a recent meta-analysis that included 9000 patients, the median efficacy of foam sclerotherapy was 87% with a median recurrence rate of 8%.68
Darke and Baker98 investigated the number of UGFS treatment sessions required for clinical evidence of complete occlusion of varicose veins. Clinical complete occlusion rates at 6 weeks following the last treatment were as follows: 74.1% with a single treatment, 89.1% with two treatments, and 89.5% with three treatments. O’Hare et al99 found no difference in efficacy rates at 6 month in veins of less than or greater than 7 mm in diameter that were treated with UGFS. However, Myers et al96 found that treatment success was decreased in veins greater than 6 mm as compared to veins of less than 5 mm. This report also concluded success rates were higher in GSVs versus SSVs.
Multiple studies have investigated the influence of various sclerosant concentrations in relation to the efficacy rate of foam sclerotherapy. Efficacy rates were highest for POL 1.5% or STS as compared to 3% or more dilute concentrations in a study investigating saphenous veins and their tributaries.96 A similar study investigating POL for UGFS of GSVs less then 8 mm found short term (3 week) success rates of 96% and 88% in subjects receiving POL 3% foam and POL 1% foam, respectively (difference was not statistically different). After 2 years, the success rates were 69% in patients treated with POL 3% foam and 68% in those treated with POL 1% foam.88 Ceulen et al100 initially found a significant difference in efficacy rates of UGFS between 1% and 3% POL foam at 1-year follow-up (69.5% and 80.1%, respectively). However, when these subjects were followed for 2.5 years, there was no difference in efficacy between the two concentrations of POL (66.7% and 66.8%, respectively).101 No exclusion criteria were made in this study with regards to the diameter of the GSV.100,101
Side effects
A foam bolus can even occur when a small quantity of foam is injected. Hill et al75 showed that leg elevation, but not manual pressure of the SFJ, decreased the migration of foam in sclerotherapy in UGFS of the great saphenous vein. At least this could ensure that bubbles will be washed of their sclerosing molecules, but will release much bigger bubbles. Other methods to decrease foam migration include multiple, sequential injections of foam sclerosant volumes less than 0.5 mL.102 A meta-analysis investigating side effects in over 9000 patients by Jia et al68 found the median rates of deep venous thrombosis and pulmonary embolus were less than 1% each. The median values of visual changes and headache were 1.4% and 4.2%, respectively. Chest tightness and coughing occurred in less than 1%. In a large, prospective, multicenter study of foam sclerotherapy in 1025 patients the incidence of migraine was 0.78% (with aura 0.59%;.19% without aura), visual disturbance 0.68%, chest tightness 0.68%, chest tightness with visual disturbance 0.49%, deep venous thrombosis 0.98%, pulmonary embolism 0.1%, and transient ischemic attack 0.1%.103
A recent study of patients with incompetent GSVs treated with UGFS created from either carbon dioxide or room air, in a 1 : 4 sclerosant to gas dilution ratio, demonstrated decreased incidences of foam-bubble-related side effects including chest tightness (3.1% to 18%), cough (1.6% to 16%), and dizziness (3.1% to 12 %). There was a trend towards decreased visual disturbances in the carbon dioxide versus room air group (3.1% to 8.2%), though this difference was not found to be statistically significant. There were no differences in vital signs or echocardiogram (ECG) changes between the two cohorts. An overall 71.79% decrease in bubble-related side effects (as compared to room air) was attributed to the carbon dioxide foam.104 When cerebral emboli were monitored via transcranial Doppler ultrasound in patients treated with foam created from either room air or a 70% carbon dioxide/30% oxygen mixture, no statistical differences were found between these two cohorts with regard to the rate of high intensity transient signals in the middle cerebral artery.105
One can speculate that the decreased upstream foam-related side effects are attributable to the short carbon dioxide foam half-life83 and the increased diffusibility of this gas into blood in comparison to nitrogen and oxygen.81,83 In a study of 116 patients with stasis ulcers, the incidence of visual changes and cough occurred in less than 2% of patients treated with carbon dioxide created UGFS.106 In a large multicenter prospective study by Guex et al, visual disturbances occurred in 0.28% of patients treated with foam sclerotherapy created using room air.107
Intravenously injected bubbles of less than 10 µm do not produce symptoms in patients, regardless of the presence of a patent foramen ovale.108 Ceulen et al109 noted foam microbubbles created from room air appeared in the right atrium and ventricle 0.75 to 15 minutes following injection. In the small study by Morrison et al,110 foam bubbles were identified in the deep venous system and the right atrium within 10 seconds of the initial injection of foam sclerotherapy into a 1-mm telangiectatic vein. This finding was reproducible in all 15 subjects. In a related study, these bubble emboli ‘showers’ could last minutes and were present in the left side of the heart in 33% of subjects, indicating a shunt. Of interest, 57% of this group of patients showed transcranial Doppler evidence of bubble emboli.111
Four cases of transient ischemic attacks and cerebrovascular events have been reported in the literature. All patients had examples of right to left cardiac shunting, such as patent foramen ovale and atrial septal defects, discovered on further workup.103,112,113 These bubbles can appear in the middle cerebral artery in under 35 seconds.114 Treatment for foam-induced neurological events includes administration of 100% oxygen followed by immediate transfer to an emergency department and the initiation of hyperbaric oxygen therapy.113 As the dominant gas in room-air bubbles is nitrogen, foam bubble half-life is decreased as the concentration of oxygen increases and the nitrogen decreases.115 Theoretically, in the event of cerebral emboli, the osmotic diffusion of oxygen and nutrients should be able to compensate for small bubbles but not larger bubbles.116
Deep venous thrombosis is a rare occurrence in foam sclerotherapy with an incidence of 0.015%.107 Shadid et al117 recommends maintaining a high index of suspicion for the development of deep venous thrombosis when superficial thrombophlebitis occurs distant from the treatment area. Pulmonary embolus have been reported following UGFS.103,118 Infection is a rare event following foam sclerotherapy, but cases of cellulitis and Klebsiella sepsis have been reported.119
Our technique for the treatment of reticular and telangiectatic leg veins
Our classification for veins are as follows: varicose veins are torturous and larger than 4 mm, reticular veins range from 1 to 4 mm, and telangiectactic veins are less than 1 mm. For the most successful outcome of foam sclerotherapy of telangiectatic leg veins, the feeding incompetent reticular veins must be treated simultaneously. At our clinic, we initiate with treatment of refluxing veins, the largest veins, and proceed in a proximal to distal fashion. The double syringe system with room air in a 1 : 5 dilution (1 part sclerosant solution: 4 parts gas) is our preferred method for foam creation. For reticular veins between 1 and 3 mm, we prefer STS 0.25% or, alternatively, POL 0.25% to 0.5%. We increase the concentration of STS to 0.5%, and POL up to 1%, for veins 3 to 5 mm in diameter. Telangiectatic leg veins are treated with liquid STS 0.25%, liquid POL 0.5%, or glycerin 72% with lidocaine 1% or without epinephrine (adrenaline). Our patients remain in the supine position without leg elevation for the duration of the treatment. Following treatment, the patient remains supine as nursing staff apply 30 to 40 mmHg compression stockings. Immediate ambulation for 15 to 30 minutes is required. For the next week, 24 hours a day (for one author RAW, only during waking hours), the patient remains in their compression stockings.120,121
Other applications of foam sclerotherapy
Ulcers
Foam sclerotherapy is also effective in the treatment of venous ulcers. Cabrera et al106 investigated carbon dioxide-POL foam in 116 patients with venous stasis ulcers and found complete resolution at 6 months in 83% of patients. Either the saphenous veins, perforator veins, or a combination were injected under ultrasound guidance. The mean time for complete resolution of an ulcer was 2.7 months. Long-standing ulcers (over 24 months), size greater than 2 cm, patient age over 65 years old, a history of venous surgery, and incompetence of the deep venous system were negative prognostic indicators.
Venous Malformations
Foam sclerotherapy can decrease the progression and size of venous malformations, including Klippel-Trénaunay syndrome.79 In a study by Yamaki et al,122 subjects with symptomatic venous malformations, including limited, infiltrating, and complex-combined variants, received ultrasound guided foam or liquid sclerotherapy. The sclerosants investigated were 1% POL foam or liquid for treatment of the superficial component and 10% ethanolamine oleate for the deep components of the venous malformation. As complete resolution of venous malformations would not be realistic in all patients in this study, patients received enough sessions until patient-perceived cosmetic satisfaction was obtained or when no further benefit was anticipated. A significant decrease in volumes of sclerosants (both POL and ethanolamine oleate) were found in the foam cohort. On 6-month follow-up duplex evaluation, increased rates of disappearance (defined as occluded and shrunken venous space) and partial recanalization (defined as partially recanalized and partially shrunken venous space) were greater in the foam group versus the liquid group (45% and 45% versus 25% and 15%).
Combination therapy
Foam sclerotherapy with saphenofemoral ligation versus high ligation, stripping, and multiple phlebectomies was evaluated by Kalodiki et al.123 With 3-year follow-up, there was no statistical difference in the recurrence rates between these two treatment regimens on Doppler ultrasound evaluation.
Foam sclerotherapy can be combined with endovenous laser ablation at the time of surgery or can be performed at follow-up visits. In a study by King, UGFS was combined with either the 980 nm or 1320 nm endovenous laser for the treatment of incompetent saphenous veins. At 1-month follow-up, treatment success was seen in 97% of patients.124 Further long-term data and controlled clinical trials will be necessary to evaluate whether combined endovenous laser ablation combined with foam sclerotherapy is superior to endovenous laser ablation alone. Any varicosities remaining at 6 weeks post procedure and distal to the site of endovenous ablation should, in our experience, be treated by foam sclerotherapy.
Contraindications
Patent Foramen Ovale
Patent foramen ovale (PFO) is present in 27% of the general population,111,112,125 and decreases in prevalence with increasing age. Transesophageal echocardiogram with contrast is the recommended screening test for the detection of PFO, but transcranial Doppler ultrasound is almost as sensitive.126 Right to left cardiac shunting can occur in the presence of a PFO, atrial septal aneurysm, or any opening between the atria, ventricles or the great vessels.127 Pulmonary arteriovenous malformations can lead to extracardiac shunting and occur in 10% of the population.111 All of the previously listed forms of shunting can result in cerebral vascular system emboli. The incidence of cryptogenic stroke is greater in patients of all ages with PFO127 as compared to the general population.128 Two prospective studies found migraines associated with an aura occur more commonly in patients with PFO.129,130
In a report by Morrison et al,110 15 patients were screened for a PFO with an ECG prior to foam sclerotherapy. Allthough echocardiogram screening revealed no PFO, intraoperative transthoracic ECG monitoring revealed the presence of four PFOs which were accentuated because of the increased visibility of foam. A 2010 study by Wright et al126 found 58.8% of patients with varicose veins (CEAP C3-5) had a right to left cardiac shunt as evidenced by the appearance of bubbles in the middle cerebral artery on transcranial Doppler (at rest or with a Valsalva maneuver) using agitated saline. Next, 61 patients with a right to left heart shunt were treated with UGFS with 1% POL foam created from a 70% carbon dioxide/30% oxygen mixture; 89% had evidence of bubble emboli in their cerebral vascular system. No patients were symptomatic throughout this study. In a study of 3259 patients treated with UGFS, 7 patients (0.21%) experienced foam-related side effects including visual disturbances, chest tightness, and migraine with aura. Of these seven patients, five tested positive on transcranial Doppler examination, indicating the presence of right to left cardiac shunting such as with a PFO.131
A known symptomatic PFO is considered an absolute contraindication for foam sclerotherapy by the 2nd European Consensus Meeting on Foam Sclerotherapy. However, the committee agreed that it is not necessary to screen for a PFO in an asymptomatic patient prior to foam sclerotherapy treatment.80
Thromboembolism and Thrombophilia
An absolute contraindication for foam sclerotherapy is the presence of an acute deep or superficial venous thrombosis. If a patient has a prior history of a deep venous thrombosus or thrombophilia, it is considered a relative contraindication. These patients should undergo a full workup to determine the etiology of thrombophilia, treatment for 7 days with prophylactic low molecular weight heparin, and be administered limited volumes and decreased concentrations of foam sclerotherapy.80 In the study by Wright el al,95 the incidence of deep venous thrombosis decreased when small volumes were utilized per injection and the injection was ceased and compression applied when foam was present 5 cm from the saphenofemoral or saphenopopliteal junction.
Migraine
A straightforward migraine is not considered a contraindication to foam sclerotherapy.80 However, we found that patients with history of ophthalmic migraine (see Chapter 8) where likely to have more frequently visual disturbances after foam sclerotherapy; this must be explained at the time of informed consent.
Other injection techniques
Air bolus
The air-bolus technique was first advocated by Orbach60 to ensure that sclerotherapy treatment of varicose veins occurred with minimal thrombosis. The rationale for instilling air before injecting the sclerosing solution is that clearing the vessel of blood allows undiluted contact to occur between the solution and the vessel wall. This procedure was thought to minimize the concentration and quantity of solution required to produce endothelial injury. A comparative evaluation of this technique demonstrated enhanced efficacy of treatment regardless of the type of sclerosing agent used.132 In reality, the potential complications of air embolism, as addressed in Chapter 8, are nil. A disadvantage of this technique is that air proximal to the sclerosing solution in the syringe causes compression of the solution, producing leakage of solution from the needle after depression of the plunger has stopped. This may cause extravasation of solution on needle withdrawal.
Radiologic studies have shown air-bolus injections into large varicose veins in the area of the GSV are ineffective in clearing the vessel of blood, even when 0.5 mL of air is injected immediately before the injection of contrast medium.49 In addition, the air inhibits complete and even filling of the varix both proximal and distal to the injection site. In smaller varicosities, injected air forms several bubbles and does not act as a bolus. It also moves independently of the position of the patient and is not totally under the influence of gravity. Therefore, the air-bolus technique may not be advantageous for use in treating varicose veins.
Ultrasound-guided injection
Ultrasound is a useful tool to help visualize injection of a varicose vein in patients who have deeply situated perforator veins, in the obese patient in whom the varicose vein is not easily palpable, in patients with recurrent varicose veins, and in patients with an unusual or complex anatomy that makes finding the point of maximum reflux difficult. Ultrasonic guidance alone does not compensate for lack of dexterity or experience. In fact, injection may be riskier, because of the complex reasons that necessitate its use. Earlier experience with less detailed ultrasound imaging demonstrated multiple complications, with the most severe being arterial injection with loss of significant amounts of tissue (see Chapter 8).133 The use of foamed sclerosant has also decreased the risks.
Ultrasound-guided injection was first described in 1989 and is a natural extension of the use of ultrasound in patient evaluation.134–138 The technique for injection is similar to that for standard sclerotherapy except that the needle used is usually longer and of larger a diameter. The author generally uses a 1.5-inch, 22-gauge needle, which is sufficiently large to be echogenic. Smaller needles (25-gauge) could not be easily seen with earlier Duplex ultrasound devices, but with newer high resolution devices even needles of 30-gauge diameter can be visualized.
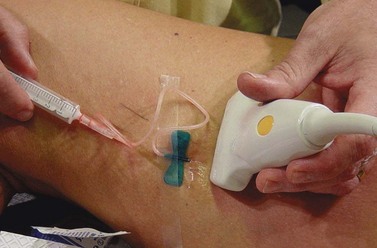
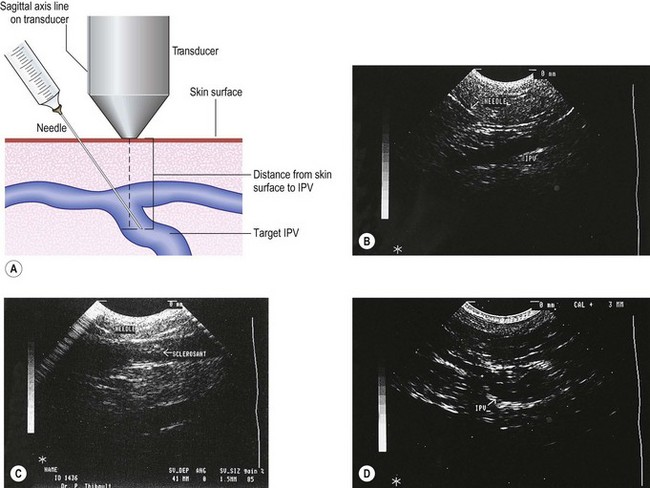
The needle is introduced into the vein open (not attached to a syringe) to ensure venous placement, because arterial injection must be avoided (Fig. 9.11A) (see Chapter 8). Injection of the foamed sclerosing solution is seen as echogenic flow (Fig. 9.11C). The injection continues until the vein goes into spasm, thrombosis occurs or foam is seen filling the vein (see Fig 9.11D, Figs 9.12 and 9.13).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


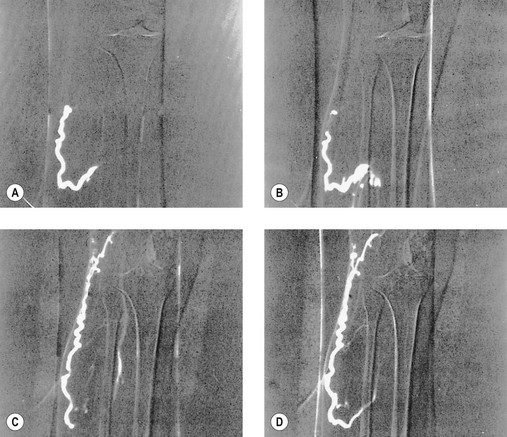
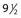 seconds and, B,
seconds and, B, 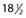 seconds after injection into a varicose vein while the patient was standing. Injected contrast media, 1.5 mL, shown, C,
seconds after injection into a varicose vein while the patient was standing. Injected contrast media, 1.5 mL, shown, C, 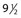 seconds and, D, 18 seconds after injection into a varicose vein while the patient was supine.
seconds and, D, 18 seconds after injection into a varicose vein while the patient was supine.