Fig. 6.1
Epidermal LBs
A paucity of cholesterol adversely affects the assembly of epidermal lamellar granules, which originate from the Golgi complex. This results in LBs with an abnormal morphology, domain separations within the stratum corneum lipids, and an increased efflux of water, quantifiable by increased transepidermal water loss (TEWL) values. It is also known that irrespective of blood cholesterol levels, the epidermis makes its own cholesterol, acting as an autonomous organ. The epidermis ramps up cholesterol synthesis when the permeability barrier is challenged, progressively returning to basal levels along with the restoration of permeability barrier function (Menon et al. 1985). Although aged skin maintains normal permeability barrier function in the basal state its ability to up-regulate cholesterol synthesis decreases in an age-dependent manner resulting in an impairment in the ability to restore permeability barrier function to normal after perturbations (Ghadially et al. 1995).
Although the autonomous nature of epidermal cholesterol synthesis was considered as one of the reasons that orally taken statins do not induce changes in skin excepting for a very small percentage of patients (who get perioral dermatitis or pigmentation abnormalities in this area). Nevertheless, several reports claim that the prolonged statin therapy could have undesirable side effects on several tissues including skin (Kiortsis et al. 2007; Hydzik and Szpak 2011) (Indeed topically applied statins in animal models, adversely affect epidermal cholesterol synthesis leading to scaly skin conditions and ultrastructural correlates of defective stratum corneum lipid organization (Fig. 6.2) with increased rates of TEWL (Feingold et al. 1991)). Again, initially an inhibition of cholesterol synthesis plays a role in the abnormal structure and function of stratum corneum, but at later time points, due to a compensatory increase in epidermal HMG-CoA reductase activity, cholesterol synthesis returns to normal. However, a concomitant increase in fatty acid synthesis results in an excess of fatty acids that alter the molar ratio of the stratum corneum lipids resulting in barrier abnormalities.
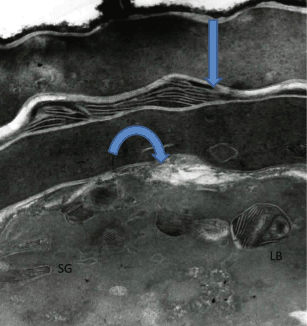
Fig. 6.2
Abnormal SC lipid organization
The authors have done some of the early and basic studies on impact of epidermal cholesterol metabolism on skin barrier function. Presently, we summarize the vast amount of work that our group and others have done in the last couple of decades that has contributed to the current state of understanding of epidermal cholesterol metabolism and skin barrier.
Unraveling the Significance of Cholesterol for Skin Permeability Barrier Function
Considered on a weight basis, mammalian epidermis is a very active site of cholesterol synthesis (Feingold et al. 1983). Within the cell, cholesterol is synthesized in the endoplasmic reticulum that contains the rate limiting enzyme for sterologenesis—HMG-CoA reductase. The significance of cholesterol for the stratum corneum permeability barrier is quite evident from the fact that the “mortar lipids” are made up of equimolar ratios of cholesterol, ceramides, and free fatty acids (Elias and Menon 1991). Studies on rodents, following acute permeability barrier disruption, demonstrated a marked and rapid increase in cholesterol synthesis (Feingold 1991). Additionally, there was an upregulation of mRNA and protein level as well as the activity of HMG-CoA reductase, a key rate-limiting enzyme in the cholesterol synthetic pathway (Jackson et al. 1992; Proksch et al. 1992, 1995). Barrier disruption also results in a notable increase in the percentage of HMG-CoA reductase in the active dephosphorylated form and this increase in activity is seen as early as 15 min after permeability barrier disruption (Proksch et al. 1990). It was also found that the extent of barrier disruption (reflected in the TEWL values) required to activate the enzyme by dephosphorylation is less than that required to increase the enzyme protein levels. The increase in HMG-CoA reductase occurs in both the upper and lower epidermal cell layers (Proksch et al. 1990). Expression of other key enzymes in the cholesterol synthetic pathway, such as HMG-CoA synthase, farnesyl diphosphate synthase, and squalene synthase, also increase after acute permeability barrier perturbation (Harris et al. 1997). However, when an occlusive membrane experimentally “restored” the permeability barrier towards normal following barrier disruption, it inhibits the increase in epidermal cholesterol synthesis as well as the mRNA levels of the cholesterol synthetic enzymes (Menon et al. 1985; Proksch et al. 1990). However, when a semipermeable membrane replaces the occlusive cover, the cholesterol synthetic response goes on unabated. These results indicate that the increase in cholesterol synthesis is an adaptive response to barrier disruption, rather than a nonspecific injury response.
Experiments with enzyme inhibitors that inhibited cholesterol synthesis showed the significance of epidermal cholesterol synthesis in permeability barrier homeostasis. Topical application of statins (inhibiting HMG-CoA reductase activity) blocked the characteristic upregulation in epidermal cholesterol synthesis that followed acute barrier disruption resulting in a delay in the recovery of permeability barrier function (Feingold et al. 1990). Interestingly, topical application of statins did not block the first wave of secretion of the nascent LBs. However, the reappearance of LBs in stratum granulosum cells (i.e., the synthesis of new LBs) is delayed and the newly synthesized LBs display abnormal internal contents (Zetterstein et al. 1998). In addition, the lamellar lipid organization of the stratum corneum barrier is also altered in appearance, indicating that an abnormality of mortar lipids is the underlying cause of the permeability barrier defect (Fig. 6.2). Topical application of cholesterol or its intermediary compound mevalonate (formed by HMG-CoA reductase) overcomes these effects demonstrating that the statin-induced defects are not nonspecific toxic effects of topical inhibitors but rather are due to a decrease in cholesterol (Feingold et al. 1990). These studies demonstrate the crucial role of epidermal cholesterol synthesis in providing cholesterol for permeability barrier homeostasis.
Further demonstrating the importance of epidermal cholesterol synthesis for permeability barrier function are studies in mice, which are deficient in 3β-hydroxysterol-Δ24, the enzyme that catalyzes the conversion of desmosterol to cholesterol. These mice fail to generate cholesterol in the epidermis, but they have an abundance of desmosterol. However, they die soon after birth due to lack of a functional permeability barrier (Mirza et al. 2006).
The potential role of extracutaneous cholesterol is suggested by the observation that inhibiting de novo cholesterol synthesis in the epidermis results in only a modest delay in the restoration of barrier function to normal. This suggests that extracutaneous cholesterol could provide the cholesterol needed for barrier restoration. Both LDL receptors and SR-B1 receptors are expressed in the epidermis and these receptors would allow for uptake of lipoproteins that contain cholesterol. Notably, the expression of both LDL receptors and SR-B1 receptors increases with permeability barrier disruption. Moreover, this increase can be prevented by occlusion that artificially restores barrier function to normal indicating that the increase in LDL receptor and SR-B1 receptor expression is linked to permeability barrier function.
Another aspect of cholesterol metabolism concerns membrane transporters such as ABCA1, which are responsible for cholesterol efflux from cells. ABCA1 plays a major role in cholesterol efflux providing a mechanism for regulating cellular cholesterol levels. Expression of ABCA1 has been documented in both undifferentiated and differentiated keratinocytes as well as the upper and lower layers of epidermis (Jiang et al. 2010). The authors also found that ABCA1 expression is decreased following acute barrier disruption, perhaps leading to reduced cholesterol efflux, allowing the cells to utilize cholesterol to meet the demands of increased LB synthesis and recovery of permeability barrier function. In addition to ABCA1, ABCG1 is also expressed in cultured human keratinocytes and murine epidermis, and it is known to be induced during the process of keratinocyte differentiation, with increasing levels towards the outer epidermis. It is regulated by liver X receptor (LXR) and PPAR activators as well as by cellular sterol levels. Moreover, acute permeability barrier disruption increases the expression of ABCG1 (Jiang et al. 2010). While ABCG1 had been thought to act at the plasma membrane to efflux cholesterol, Tarling and Edwards (2011) showed that ABCG1 also localizes to the intracellular endosomes. The transport of cholesterol to epidermal LBs may be partially mediated by ABCG1, as ABCG1 KO mice display defective (empty appearing) LBs, and a reduction in SC lamellar membranes (Jiang et al. 2010). However, permeability barrier function is normal in these animals indicating that ABCG1 is not essential for the formation of LBs suggesting that there may be multiple mechanisms for cholesterol transport into LBs.
Other barriers present in the epidermis are also influenced by the cholesterol synthetic pathway. For example, CoQ 10 is an important antioxidant that functions in the oxidant barrier of skin and its role in skin health is very well appreciated (Shindo et al. 1994). CoQ 10 is derived from the same pathway that leads to cholesterol and drugs that inhibit HMG-CoA reductase activity would also adversely affect CoQ 10 production (Mabuchi et al. 2005). In the skin, the reduced form of CoQ10 (ubiquinol) acts as an antioxidant with tenfold higher levels in the epidermis than in the dermis (Shindo et al. 1994). Inoue et al. (2008) found that normal human keratinocytes in vitro, in the presence of CoQ 10 showed decreased IL-6 production following UVB exposure. Additionally, when fibroblasts that were pretreated with CoQ 10 were exposed to conditioned media from UVB exposed keratinocytes, the characteristic response of MMP-1 production was also downregulated. In a clinical study, daily applications of a 1 % CoQ10 cream for 5 months were also reported to reduce the wrinkle score grade (Inoue et al. 2008), which they attributed to a reduction in Matrix Metaloprotease (MMP) production (Inoue et al. 2008). Such studies underlie the significance of the cholesterol synthetic pathway.
Additionally, within the epidermal compartment that houses melanocytes, cholesterol also has an important role in another facet of the barrier—namely melanogenesis. Schallrueter et al. (2009) showed that human epidermal melanocytes have the capacity of cholesterol signaling via Apo-B/LDL receptor and that cholesterol under in vitro conditions increases melanogenesis. Although the significance of melanin as a UV barrier has often been disputed, the microparsol of melanosomes that cap the basal keratinocyte nuclei has long been considered to be crucial for DNA protection in these cells. Active melanocytes no doubt have a role in epidermal barrier, as permeability barrier recovery under experimental conditions in mice and humans is more efficient in darkly pigmented skin (Reed et al. 1995).
Additionally, dehydrocholesterol is a precursor for vitamin D, whose importance in health is very well recognized. UV induced synthesis of vitamin D makes a major contribution to total body vitamin D stores. In fact if exposed to sufficient sunlight one does not have to ingest vitamin D as the skin is capable of making all the vitamin D that is required. In the epidermis 1, 25 dihydroxy vitamin D binds to the vitamin D receptor, which heterodimerizes with RXR to control the transcription of several genes that regulate the differentiation of keratinocytes and permeability barrier function. Considering all of the important roles that the cholesterol biosynthetic pathway assumes in keratinocytes the predominantly autonomous nature of epidermal cholesterol synthesis appears to be a huge selective advantage.
Effects of Aging
Like many other functions that decline with age, epidermal cholesterol synthesis is decreased in the elderly and this decrease is associated with a decrease in HMG-CoA activity. Permeability barrier function (measured by TEWL) appears normal in aging or actually better than normal, often attributed to the decreased microcirculation of the aged skin. Whatever be the case for normal TEWL in aged skin, there is a delay in the recovery of permeability barrier function following acute perturbation, in both aged mice as well as humans (around 75 years of age). This is associated with a decrease in extracellular lipids of the stratum corneum, which is due to a decrease in epidermal cholesterol synthesis (Ghadially et al. 1995). The LB synthetic and secretory responses are blunted in aged skin, but topical application of mevalonic acid or cholesterol can correct the abnormality demonstrating the key defect is the inability of aged skin to make sufficient amounts of cholesterol (Haratake et al. 2000).
Yet another cholesterol connection with ageing changes in skin could be related to its role in modulating metalloproteinases—which play a significant role in dermal changes manifested as wrinkles, especially in photoaging. Quan et al. (2009) have shown that following experimental UV irradiation of human skin, all three UV irradiation inducible MMPs were produced primarily in the epidermis. Thus, in photoaging, the epidermis plays a significant role in inducing dermal changes. Additionally, cholesterol treatment of fibroblasts has been reported to increase expression of tissue inhibitor of metalloproteinases (TIMP) (Tyagi et al. 1997), and a declining cholesterol synthesis in aging skin could also modulate the activity of metalloproteinases, enhancing the dermal changes. A recent paper (Kim et al. 2010) suggests that cholesterol depletion leads to inhibition of TIMP-2 which in turn leads to the conversion of proMMP2 into active MMP2 in human dermal fibroblasts. Therefore, it appears that the presence of cholesterol may also be fundamental for healthy extracellular matrix of the dermis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






