Breast Cancer Screening and Diagnosis, Including the Surgically Altered Breast
Toni Storm
Allen Gabriel
History
Breast cancer is common, affecting about 1 in 8 women (12.4%) with over 260,000 new cases per year in the United States (1). As of January 2019, there were more than 3.1 million women either with a history of breast cancer or being treated for breast cancer in the United States alone (2). Less than 1% of breast cancers develop in men. Not including benign breast biopsies and cosmetic breast surgery, there are over a half a million breast cancer–related surgeries performed per year in the United States (3). In spite of all these breast cancer surgeries, breast imaging recommendations remain controversial with the United State Preventative Services Task Force (USPSTF) recommendations differing, in varying degrees from most of our other guiding bodies; American College of Radiology (ACR), American Cancer Society (ACS), American Society of Breast Surgeons (ASBrS), and Society of Surgical Oncology (SSO) (4).
In the United States, the 5-year survival rates for women with breast cancer have improved from 75% in 1975–77 to 90% in 2003–09 (5). The risk of distant or metastatic disease and death increases with both tumor size and number of axillary lymph nodes involved (6,7,8,9). While mammography is not a perfect test and may be particularly insensitive at detecting breast cancer among selected groups of patients such as those with very dense breast, extensive scarring from previous interventions (such as surgery and radiation), or those with a subtype of malignancy which is often harder to detect on imaging such as invasive lobular carcinoma, it remains effective at finding smaller tumors, before they are palpable (10,11,12,13). When measuring the benefit of an intervention as when measuring the harm, survival should not be the only measure of efficacy. After tumor size and lymph node involvement, survival is strongly influenced by tumor-related factors such as hormone receptor, human epidermal growth factor receptor 2 (HER-2) status, lymphovascular invasion (LVI), and grade (7,8,9,10). Screening mammography is effective at finding many cancers early, before they are palpable, and thereby reducing the number of women with cancers of advanced size and stage. Finding cancers at an earlier stage allows for better outcomes, more lives saved, and potential for both less extensive surgery and no or less extensive chemotherapy.
Imaging in Women 40 to 70
In part, the controversy over when to initiate screening mammography arises secondary to the fact that breast cancer is not only common, it is potentially deadly, but not uniformly so. Breast cancer represents about 20% of all cancers (men and women) and is the second most common cause of cancer death among women over all (14). This number continues to improve in women over 50, with breast cancer deaths having dropped by approximately 37% between 1989 and 2015 in this population (15) (Table 2-1, USPSTF Grades). However, in women under 50, the death rate has remained steady since 2007. Data has also shown that younger women are more likely to develop more aggressive malignancies (HER-2+ and hormone receptor negative) with higher risk of both distant and local recurrence (16,17,18,19,20,21,22,23,24).
We often think of breast cancer as a disease of the elderly, which is not untrue (Figs. 2-1 and 2-2). However, this is a very limited picture of the true impact and distribution of the disease. Breast cancer is most commonly diagnosed in middle-aged women with a broad distribution extending to the young adult and the very old. As stated earlier, the “lifetime” risk of developing breast cancer is one in eight women, with 25.9% of all breast cancers diagnosed between the ages of 55 and 64 with an average age of 62 at diagnosis (Fig. 2-2). However, it is extremely important to note that there is an almost equal distribution 10 years above and below this with, 20.4% of women diagnosis between 45 and 54 years of age and 24.1% diagnosis between 65 and 74 years of age. Context remains extremely important as seen in Figure 2-3, with lifetime risk seen to be highest in women aged 80,
however, the age at which the largest number of women are diagnosed is 62 (Fig. 2-3). Further, survival improves with earlier stage at diagnosis; 5-year survival for stage I breast cancer is 98.7% compared to 27% for metastatic disease (25).
however, the age at which the largest number of women are diagnosed is 62 (Fig. 2-3). Further, survival improves with earlier stage at diagnosis; 5-year survival for stage I breast cancer is 98.7% compared to 27% for metastatic disease (25).
TABLE 2-1 The USPSTF Average Risk Breast Cancer Screening Guidelines, 2015 | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
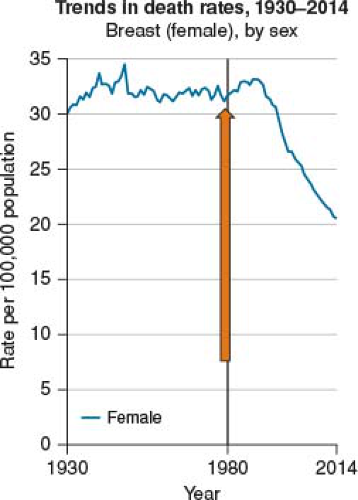 FIGURE 2-1 Declining death rate 37% between 1989 and 2015 for women over 50. (Data from American Cancer Society SEERS Data, NIH 2016.) |
We also know that the vast majority of breast cancers are sporadic at 85% to 90% (25,26) thus, lacking a family history cannot be interpreted as protective. Using lack of a family history to exclude women under 50 from screening mammography leaves a large and vulnerable group of “average-risk” women with a misimpression that they are somehow safe and will not benefit from mammography. The United States Preventative Services Task Force (USPSTF) is an independent panel of primary care physicians and epidemiologists funded, staffed, and appointed by the U.S. Department of Health and Human Services that make recommendations for clinical preventative services. Their original recommendations, set forward in 2002, used a meta-analysis of the eight large prospective mammography trials designed to assess the effectiveness of mammography in reducing breast cancer mortality but only included data from seven (27). All the trials had limitations but the USPSTF excluded the Edinburgh study from the analysis, secondary to imbalance between control and screened group. USPSTF concluded: “mammography reduced breast cancer mortality among women 40 to 74 years of age with a greater benefit in women greater than 50” and at that time continued to recommend mammograms annually starting at age 40.
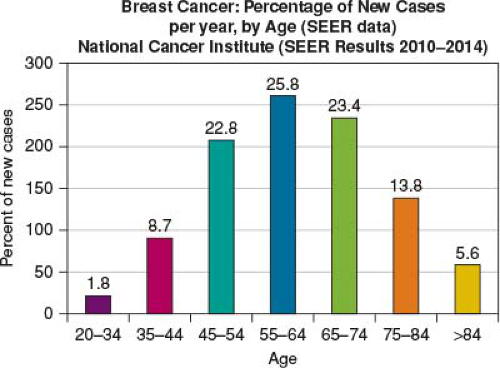 FIGURE 2-2 Breast cancer: percentage of new cases per year, by age. (Data from NIH [National Institutes of Health], SEERS [Surveillance Epidemiology and End Results Program] 2019.) |
In 2009, the USPSTF updated their analysis to include data from the Age trial from the United Kingdom that randomized women 39 to 41 to annual screening mammography until age 48 (28). The purpose of their evaluation was to “determine the effectiveness of mammography screening in decreasing breast cancer mortality among average-risk women aged 40 to 49 years and 70 years or older, the effectiveness of clinical breast examination and breast self-examination, and the harms of screening.” They published their results in Annals of Internal Medicine November 2009. The study used film and digital mammography and The Task Force again found a 15% reduction in breast cancer mortality in favor of screening with an even greater benefit for women over 60. They reported the false positive rate highest in women aged 40 to 49 with the highest rate of additional imaging and unnecessary biopsies in this age group. Secondary to their concerns for the harm–benefit ratio, they changed their recommendations to consider starting mammographic
screening at age 50. Further they found no benefit for clinical breast examination and self-breast examination was considered harmful.
screening at age 50. Further they found no benefit for clinical breast examination and self-breast examination was considered harmful.
In their conclusion they stated that “Our meta-analysis of mammography screening trials indicates breast cancer mortality benefit for all age groups from 39 to 69 years, with insufficient data for older women. False-positive results are common in all age groups and lead to additional imaging and biopsies. Women aged 40 to 49 years experience the highest rate of additional imaging, whereas their biopsy rate is lower than that for older women. Mammography screening at any age is a trade-off of a continuum of benefits and harms. The ages at which this trade-off becomes acceptable to individuals and society are not clearly resolved by the available evidence” (29).
Of very significant import is that the USPSTF primary concern with mammography was not its ability to detect cancers earlier than would be found without imaging and thereby prevent breast cancer–related deaths, but rather harm of imaging outweighing the benefit based on unnecessary imaging and biopsies as well as costs. With this in mind, note that their studies used plain films and digital mammography. We now have 3D breast tomosynthesis, rapidly becoming the standard of care, which has shown a reduction in false positives by 17.1% and increased rate of detection of breast cancers by 33.9% over standard digital mammography (30).
If we combine (1) the improved diagnostics of tomosynthesis with fewer false positives and better detection rate, (2) ≥15% decrease in mortality with early diagnosis through mammography, (3) the fact that women under 50 account for approximately 24% of breast cancers diagnosed, (4) younger women tend to have more aggressive disease which will progress rapidly and cost more to treat, and (5) do not qualify for screening mammography by USPSTF guidelines, we can make a very compelling argument to change the recommendations on screening mammography to start at age 40.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree