51. Breast Anatomy
Melissa A. Crosby, Glyn Jones
EMBRYOLOGY, DEVELOPMENT, AND PHYSIOLOGY
EMBRYOLOGY
■ The breast is ectodermally derived.
■ From week 8-10 of embryologic development, breast growth begins with differentiation of cutaneous epithelium of pectoral region.
■ In week 6, milk ridge develops extending from axilla to groin.
■ From week 7 of gestation to birth, mammary anlage on chest wall develops into an epithelial bud with 15-20 ducts, and nipple develops into circular smooth muscle fibers.
■ First 7 weeks after birth, clear fluid similar to colostrum (“witch’s milk”) containing water, fat, and cellular debris may be secreted from the neonatal breast, stimulated by maternal hormones.
■ Normal breast development in anterolateral pectoral region at level of fourth intercostal space
■ Supernumerary breasts (polymastia) and nipples (polythelia) can occur along milk ridge.
• Most common location for polymastia is lower left chest wall below the inframammary crease.
• Polythelia is the most common congenital breast anomaly, occurring in 2% of the population.
■ Abnormal regression of milk line can lead to underdevelopment of breasts (hypoplasia).
■ Complete absence of breast (amastia) usually associated with hypoplasia of ipsilateral pectoralis musculature and chest wall (Poland syndrome).
DEVELOPMENT
■ Puberty begins at 10-12 years of age as a result of hypothalamic gonadotropin-releasing hormones secreted into the hypothalamic-pituitary portal venous system.
■ Anterior pituitary secretes follicle stimulating hormone (FSH) and luteinizing hormone (LH).
■ FSH causes ovarian follicles to mature and secrete estrogens.
■ Estrogens stimulate longitudinal growth of breast ductal epithelium.
■ As ovarian follicles become mature and ovulatory, the corpus luteum releases progesterone, which, in conjunction with estrogen, leads to complete mammary development.
■ Stages of breast development described by Tanner1:
• Stage 1: Preadolescent elevation of nipple only; no palpable glandular tissue or areolar pigmentation
• Stage 2: Presence of glandular tissue in the subareolar region; nipple and breast project as single mound
• Stage 3: Further increase in glandular tissue with enlargement of breast and nipple but continued contour of nipple and breast in single plane
• Stage 4: Enlargement of areola and increased areolar pigmentation with secondary mound formed by nipple and areola above level of breast
• Stage 5: Final adolescent development of a smooth contour with no projection of the areola and nipple
■ Normal variants in breast development
• Infantile hyperplasia of breast
► Result of transplacental estrogen from maternal-placental unit
► Occurs in both sexes and may be associated with secretion of colostrum
► Found in more than half of newborns
• Pubertal gynecomastia
► Occurs in 70% of boys
► May be unilateral or bilateral
► Tender
► Can persist for up to 2 years
• Premature thelarche
► Breast development beginning before 8 years of age in girls without other signs of puberty or skeletal maturation
► Most often bilateral but can be unilateral
► Usually noted with first 2 years of life and ends after 3-5 years
• Delayed maturation
► Absence of breast development by 14 years of age in absence of chronic illness or endocrine abnormality
► Family history of delayed maturation typical
► Because relatively uncommon, need to rule out primary ovarian failure by testing for abnormal gonadotropin levels
MENSTRUAL CYCLE
■ Premenstrual: Estrogen peak, breast engorgement, breast sensitivity
■ Follicular phase (days 4-14): Mitosis and proliferation of breast epithelial cells
■ Luteal phase (days 5-28): Progesterone levels rise, mammary ducts dilate, and alveolar epithelial cells differentiate into secretory cells; estrogens increase blood flow to breast
■ Menstruation: Breast involution and decrease in circulating hormones
■ Breast engorgement and tenderness (at a minimum 5-7 days after menstruation): Palpation is most sensitive for detecting masses and most comfortable for patient at this time.
PREGNANCY AND LACTATION
■ Marked ductal, lobular, and alveolar growth occurs under influence of estrogen, progesterone, placental lactogen, prolactin, and chorionic gonadotropin.
■ First trimester: Estrogen influences ductal sprouting and lobular formation, early to late breast enlargement ensues, superficial veins dilate, and pigmentation of nipple-areola complex (NAC) increases.
■ Second trimester: Lobular events predominate under influence of progestins, and colostrum collects within the lobular alveoli.
■ Third trimester: By parturition, breast size triples from vascular engorgement, epithelial proliferation, and colostrum accumulation.
■ Withdrawal of placental lactogen and sex hormones with delivery results in breast being predominantly influenced by prolactin.
■ Anterior pituitary secretion of prolactin influences milk production and secretion.
■ Posterior pituitary secretion of oxytocin leads to breast myoepithelial contraction and milk ejection.
■ Prolactin and oxytocin secretion is stimulated by nursing infant’s tactile stimulation of nipple.
■ Postlactational involution occurs during the 3 months after cessation of nursing; regression of extralobular stroma is a primary feature.
MENOPAUSE
■ Involves loss of glandular tissue and replacement with fat
■ Some lobules remain, but postmenopausal breast consists mainly of fat, connective tissue, and mammary ducts.
VASCULAR SUPPLY2 (Fig. 51-1)
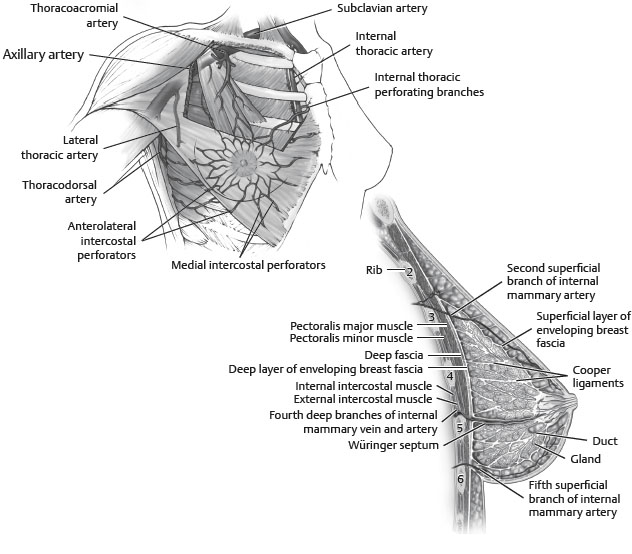
Fig. 51-1 Vascular supply of breast.
ARTERIAL SUPPLY
■ Skin
• Receives blood supply from subdermal plexus, which communicates through perforators with underlying deeper vessels supplying the breast parenchyma.
■ Parenchyma
• Supplied by:
► Perforating branches of internal mammary artery
► Lateral thoracic artery
► Thoracodorsal artery
► Intercostal perforators
■ Nipple-areola complex
• Receives both parenchymal and subdermal blood supply
VENOUS DRAINAGE
■ Follows the arterial supply
INNERVATION2 (Fig. 51-2)
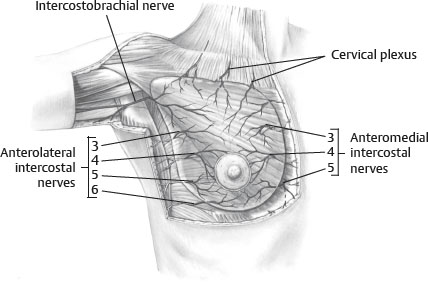
Fig. 51-2 Innervation to breast.
■ Dermatomal in nature
■ Derived from the anterolateral and anteromedial branches of the thoracic intercostal nerves T3-5
■ Supraclavicular nerves from lower fibers of cervical plexus also provide innervation to the upper and lateral portions of the breast.
■ Nipple-areolar sensation is derived from the anteromedial and anterolateral T4 intercostal nerve.
■ Intercostal brachial nerve courses across axilla to supply upper medial arm and is often injured during axillary dissection, resulting in anesthesia and paresthesia.
ANATOMIC STUDIES
■ Schlenz et al3
• 28 unilateral breast dissections in female cadavers
• Found consistent innervation of the NAC by the anterior and lateral cutaneous branches of the third through fifth intercostal nerves
• Lateral cutaneous branch (LCB) supplied innervations through posterior innervations of the nipple in 93%.
► Fourth LCB provided posterior innervations in 93% of cases and was the only source 79% of the time.
• Anterior cutaneous branch (ACB) had superficial course to supply medial aspect of NAC.
► Third and fourth ACB combined to provide innervations in 57% of cases.
• 28 anatomic dissections and 14 arterial injection studies of female cadavers
• Defined a “brassierelike” connective tissue suspensory system
• Found neurovascular supply to the nipple runs along this well-defined suspensory apparatus
► Vertical ligaments originated from the pectoralis minor (laterally) and sternum (medially)
• Defined parenchymal borders and carried corresponding neurovascular structures
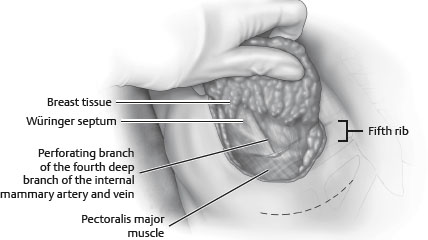
• Horizontal septum originated from the pectoral fascia along the fifth rib: Würinger septum (Fig. 51-3).