Botulinum toxin is a safe and effective treatment option for axillary hyperhidrosis. Although its pathophysiology is not clear and somewhat controversial, the beneficial effect of neuromodulators in inhibiting localized sweating temporarily is well known. Before the procedure, correct identification of the affected area is mandatory to avoid wastage of drug and neglect of target areas, and to enhance efficacy, as the hyperhidrotic location may not match the hairy axillary region. Utilization of this medication, such as dilution and injection techniques, depends on medical experience and may have some variations, including methods to make the procedure as painless as possible.
Key points
- •
Botulinum toxin has been proved to be safe and effective for the treatment of axillary hyperhidrosis.
- •
Although its pathophysiology continues to be controversial, the beneficial effect of type-A neuromodulators in temporarily inhibiting localized sweating supports a level A recommendation from evidence-based review.
- •
Before the procedure, the correct identification of the affected area is mandatory to avoid wastage of drug and neglect of target areas, and to enhance efficacy, as the hyperhidrotic location may not match the hairy axillary region.
Introduction
Axillary hyperhidrosis is a disease that affects the social and occupational lives of many people on all continents. Axillary hyperhidrosis begins during the teenage years and equally affects men and women. When associated with axillary malodor it is known as bromhidrosis.
The pathophysiology of primary focal hyperhidrosis is not well understood. It can result from hyperstimulation of eccrine and, possibly, apoeccrine sweat glands.
Eccrine glands are distributed over almost the entire body surface and are most numerous on the palms, soles, forehead, axillae, and cheeks. Innervated by cholinergic postganglionic sympathetic nerve fibers, they excrete sweat and contribute to regulation of body temperature. When comparing patients with excessive sweating with normal controls, histologic studies have not shown any morphologic alterations or increase in the number or size of the sweat glands. However, preliminary findings of a recent study suggest that the eccrine gland’s secretory clear cell exercises a main role in fluid transport (the only one equipped with cotransporter and aquaporin channels), and is likely the source of excessive sweating in this form of hyperhidrosis.
Apocrine glands are stimulated by epinephrine and norepinephrine, and are specifically localized at the urogenital regions and the axillae. These glands produce a viscid secretion that can become malodorous as a result of bacterial breakdown.
Sato and colleagues described apoeccrine glands in 1989 as having morphologic characteristics of both eccrine and apocrine types. According to these investigators, they correspond to 10% to 45% of all axillary glands and respond to cholinergic stimuli, and intensely so to epinephrine and isoproterenol infusion. However, recent histologic studies have failed to show evidence of apoeccrine glands in the tissues of the axillary region investigated. The existence of these glands remains controversial.
Introduction
Axillary hyperhidrosis is a disease that affects the social and occupational lives of many people on all continents. Axillary hyperhidrosis begins during the teenage years and equally affects men and women. When associated with axillary malodor it is known as bromhidrosis.
The pathophysiology of primary focal hyperhidrosis is not well understood. It can result from hyperstimulation of eccrine and, possibly, apoeccrine sweat glands.
Eccrine glands are distributed over almost the entire body surface and are most numerous on the palms, soles, forehead, axillae, and cheeks. Innervated by cholinergic postganglionic sympathetic nerve fibers, they excrete sweat and contribute to regulation of body temperature. When comparing patients with excessive sweating with normal controls, histologic studies have not shown any morphologic alterations or increase in the number or size of the sweat glands. However, preliminary findings of a recent study suggest that the eccrine gland’s secretory clear cell exercises a main role in fluid transport (the only one equipped with cotransporter and aquaporin channels), and is likely the source of excessive sweating in this form of hyperhidrosis.
Apocrine glands are stimulated by epinephrine and norepinephrine, and are specifically localized at the urogenital regions and the axillae. These glands produce a viscid secretion that can become malodorous as a result of bacterial breakdown.
Sato and colleagues described apoeccrine glands in 1989 as having morphologic characteristics of both eccrine and apocrine types. According to these investigators, they correspond to 10% to 45% of all axillary glands and respond to cholinergic stimuli, and intensely so to epinephrine and isoproterenol infusion. However, recent histologic studies have failed to show evidence of apoeccrine glands in the tissues of the axillary region investigated. The existence of these glands remains controversial.
Botulinum toxin
Intracutaneous injections of botulinum toxin (BoNT) have been used as a treatment for focal hyperhidrosis since 1996 with safety, efficacy, and high levels of patient satisfaction. Two types of botulinum toxins, BoNT type A (BoNT-A) and BoNT type B (BoNT-B), were studied in axillary hyperhidrosis, and both demonstrated effectiveness in temporarily inhibiting sweating, although acting at different target sites. BoNT-A binds to and cleaves the 25-kDa synaptosomal-associated protein (SNAP-25), whereas BoNT-B acts on the vesicle-associated membrane protein (VAMP or Synaptobrevin), both blocking the release of acetylcholine from cholinergic neurons that innervate sweat glands.
The use of BoNT-A for the treatment of axillary hyperhidrosis was approved in 2004 by the US Food and Drug Administration (FDA), since then a multitude of studies have confirmed its efficacy, beneficial effects, and paucity of side effects.
There are many commercial available BoNT-A products available worldwide. The formulations are not identical and present individual potencies, making caution necessary to ensure proper use. In April 2009, the FDA established drug names to reinforce these differences, summarized in Table 1 .
| Botulinum Toxin | Trade Name | Origin |
|---|---|---|
| OnabotulinumtoxinA (OnaA) | Botox | (Allergan, Irvine, CA, USA) |
| AbobotulinumtoxinA (AboA) | Dysport | (Ipsen Biopharm, UK) in USA, Europe, and Latin America |
| BoNT-A | Prosigne | (Lanzhou, China) in Asia and Latin America |
| BoNT-A | Neuronox | (Medytox, South Korea) in Asia, Botulift in Latin America |
| IncobotulinumtoxinA (IncoA) | Xeomin | (Merz Pharma, Germany) in Canada, Germany, USA, Latin America |
| BoNT-A | PureTox | (Mentor Corp, Santa Barbara, CA, USA) uncomplexed BoNT-A. Phase III studies |
There is no globally accepted exact ratio among the different formulations. Reviewing the related published literature, the most commonly accepted dose correlation among products are: 1 U onabotulinumtoxinA (OnaA) = 1 U incobotulinumtoxinA (IncoA) = 1 U BoNT-A (Lanzou) = 1 U BoNT-A (Medytox) = 2,5–3 U abobotulinumtoxinA (AboA).
The available BoNT-B (rimabotulinumtoxinB [RimaB]) products are Neurobloc in the European Union and Myobloc in the United States. Unlike BoNT-A, it is not commercially available worldwide, and probably for this reason a limited number of studies of axillary hyperhidrosis being treated with this toxin type have been published. The literature found describes side effects related to distant spread of the toxin, such as dry eyes and dry mouth, which are not commonly described after the use of BoNT-A. The dose correlation between BoNT-A and BoNT-B varies from 20 to 100 U of RimaB to 1 U of OnaA.
A recent evidence-based review of hypersecretory disorders that searched for botulinum toxin as a treatment of axillary hyperhidrosis found 2 Class I (prospective, randomized, controlled, and with masked outcome assessment clinical trial with strict requirements) studies (1 with OnaA and 1 with AboA ) and 5 Class II (similar to Class I trials but lacking 1 or more of the required criteria) studies. The investigators concluded that the evidence supports a level A recommendation for BoNT-A in general and a level B recommendation for OnaA and AboA individually, whereas RimaB and IncoA received a level U recommendation (insufficient data) for axillary hyperhidrosis.
Some studies have compared the use of different toxins for the treatment of axillary hyperhidrosis.
Studies Comparing BoNT-A Products
Kalner performed a prospective same-patient comparison between OnaA in one axilla and AboA in the other, using a conversion factor of 1 U OnaA to 3 U AboA. She noted that OnaA resulted in a faster onset of action, within 1 week, versus 2 weeks for AboA. She also observed a longer duration of benefit (9 months), whereas the axilla treated with AboA maintained the results for 6 months. In another comparative study performed in 2007 on 10 patients, Talarico-Filho and colleagues did not find statistically significant differences in the onset of sweating reduction or the duration of benefit using the same conversion factor.
In a double-blind comparative study of 46 patients, Dressler injected 50 U OnaA in one axilla and 50 U IncoA in the contralateral axilla. Both 100-U/vial products were reconstituted in 10 mL of saline (10 U/mL). He found no difference in efficacy, onset of action, duration, or side effects between the 2 formulations.
Studies Comparing BoNT-A and BoNT-B Products
In 2011, Frasson and colleagues treated 10 patients using 2500 U of RimaB in one axilla and 50 U of OnaA in the contralateral axilla (50 U B:1 U A). BoNT-B was more effective than BoNT-A in reducing sweating production in the affected area, with faster onset, longer duration of benefit, and higher treatment satisfaction scores. No systemic adverse effects were described. According to the investigators, their findings differed from those found in the literature because other studies used lower toxin ratios (40:1 or 20:1) and higher dilutions.
Further studies are needed to standardize the treatment while aiming at reducing side effects and improving the benefits. The toxin type will be selected at the physician’s discretion and according to its safety and product availability.
Toxin solution
A recent review about botulinum toxin handling found that “there is no standardized dilution for BoNT-A treatment of focal hyperhidrosis.” Reported dilutions found in the literature vary from 1 to 10 mL of saline for OnaA (with most physicians using between 2 and 5 mL), whereas for AboA the reconstitution volumes vary from 1.25 to 10 mL (with the use of 2.5–5 mL being the most frequent). In the only study of IncoA for hyperhidrosis, the dilution used was 10 U/mL. Table 2 summarizes the dilution volumes described in the literature.
| Toxin | Dilution Range (mL saline) | Most Commonly Used Dilution (mL) |
|---|---|---|
| OnabotulinumtoxinA | 1–10 | 2–5 |
| AbobotulinumtoxinA | 1.25–10 | 2.5–5 |
| IncobotulinumtoxinA | 1–10 | 10 (1 paper) |
The authors prefer to reconstitute the 100-U vial of OnaA (Botox) in 2 mL saline, achieving a dose of 50 U/mL.
The same article previously quoted also mentions that different substances can be added to the toxin solution with no harm to the toxin, such as hyaluronidase, lidocaine, and epinephrine.
Among these substances, the most interesting for axillary treatment is lidocaine. A recent double-blind, randomized, comparative study treated 8 patients with 50 U OnaA diluted in 0.5 mL saline plus 1 mL of 2% lidocaine into one axilla, and 50 U OnaA diluted in 1.5 mL saline into the other axilla. Vadoud-Seyedi and Simonart also treated 29 patients in a similar manner in 2007, with a dilution of 5 mL. Both studies showed equal effectiveness of BoNT-A reconstituted in saline or lidocaine. However, the toxin diluted in lidocaine caused less pain, and may be preferable for treating axillary hyperhidrosis.
When reconstituted with saline admixed with hyaluronidase, OnaA has its efficacy maintained after 2 weeks and shows enhanced diffusion, as observed by Goodman in 2003.
Evaluation methods
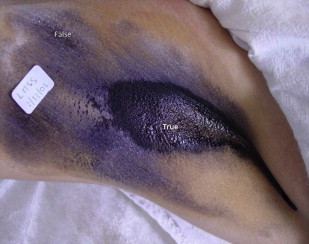
After the selection of the toxin, it is important to identify the area to be treated. The Minor iodine-starch test is a useful method to map the extension of the affected area in addition to the posttreatment residual sweating, but it does not provide accurate information on the amount of sweat produced.
The test is usually applied before any topical or regional anesthesia, and is cheap and easy to perform. The first step is to dry the affected area with an absorbent paper. Then a 3% to 5% iodine solution is applied to the underarm and neighboring region and is allowed to dry. In some patients, the continuous sweat must be wiped again just before the starch application to avoid false reactions ( Fig. 1 ). In contact with starch plus iodine, the sweat acquires a dark purple color, being clearly visible. One must be aware that the commercial povidone-iodine topical solution with 10% iodopovidone contains only 1% free iodine. Therefore, when using this agent the Minor test results might not be satisfactory.