21. Botulinum Toxin
Joshua Lemmon, Smita R. Ramanadham, Miles Graivier
DEFINITION
Botulinum toxin is a neurotoxin that is produced by Clostridium botulinum, a gram-positive anaerobic bacterium.
HISTORY AND PHYSIOLOGY1,2
BOTULISM
■ The toxin was identified as the cause of a symmetrical, neuroparalytic illness.
■ Foodborne botulism occurs from the ingestion of the neurotoxin and rarely occurs in the modern era, except in home-canned goods or rare instances.
■ Wound botulism occurs when the bacteria colonize a wound and produce the toxin, causing progressive weakness.
■ Infant botulism—the most common in the United States (100 infants/year)—results from the ingestion of clostridial spores, colonization of the gut with the bacteria, and production of toxin.
TOXINS3
■ Clostridium botulinum produces eight toxins, of which seven have paralytic properties: serotypes A through G.
• Antigenetically distinct with different sites of action4
■ Mechanism of action
• The neurotoxin acts at presynaptic nerve terminals to inhibit the release of acetylcholine, producing a chemodenervation.
• The protein consists of a heavy and light chain. The heavy chain irreversibly binds to the nerve terminal, and the toxin is internalized through endocytosis, where it renders the nerve terminal nonfunctional and blocks the release of acetylcholine into the neuromuscular junction.4
• It targets the SNAP/SNARE docking protein complex and the vesicle-associated membrane protein (VAMP) (type B toxin).
• Recovery is through two phases:
► Phase 1: Accessory terminals sprout from the affected axon to stimulate the postsynaptic target.
► Phase 2: After 28 days, the main axon teminal begins slow recovery of its acetylcholine release ability. At appromately 90 days recovery is complete.4
■ Type A was purified in crystalline form in 1946.
• In the 1970s type A was first used clinically in treating strabismus and then facial dystonias.
• Carruthers and Carruthers5 then described the first aesthetic use of toxin for the treatment of glabellar frown lines in 1992.
■ Types A and B are FDA approved for clinical use.
• Type A is approved for multiple clinical uses, including the cosmetic improvement of glabellar rhytids and crow’s-feet in patients 65 years or younger.
► Expanded to include hyperhidrosis, blepharoptosis, and cervical dystonia.
• Type B is approved for cervical dystonia.
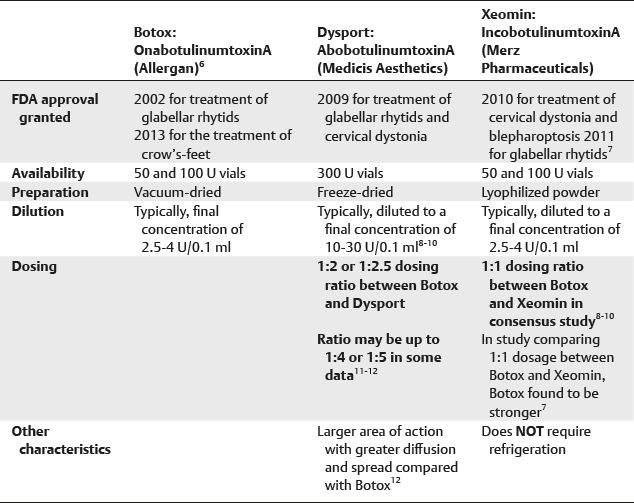
■ Three preparations of type A are available for facial cosmetic use (Table 21-1).
Table 21-1 Preparations of Type A Botulinum Toxin
INDICATIONS AND CONTRAINDICATIONS2,13
INDICATIONS
■ Despite limited FDA approval, widely used off-label to improve dynamic facial rhytids
CONTRAINDICATIONS
■ Active infection at the proposed injection site
■ Known hypersensitivity to any ingredient in the formulation, including albumin
PRECAUTIONS
■ Botulinum toxin is used cautiously in:
• Patients with neuromuscular disorders including ALS, myasthenia gravis, and Lambert-Eaton syndrome, because they may have significant side effects
• Coadministration with aminioglycoside antibiotics or other agents that interfere with neuromuscular transmission, which can potentiate the effect of type A toxin
• Pregnant women (category C): Adverse effects shown in animal pregnancy studies, but inadvertent use has not resulted in problems in humans
• Lactating patients, as it is unknown whether the toxin is excreted in human milk
• Inflammatory skin conditions are the site of planned injection
PATIENT EVALUATION
HISTORY
■ Age
■ Gender
■ Medical comorbidities
■ History of prior treatments and preferences
■ NSAID use and other medications (may increase bruising)
ANALYSIS
■ Evaluate static and dynamic rhytids in the upper and lower face.
■ Identify platsymal bands.
■ Assess brow symmetry and aperture width.
■ Identify any preexisting brow or upper lid ptosis.
OTHER CONSIDERATIONS
■ Skin quality: More toxin is generally required for thicker skin.
■ Muscle mass: Male patients tend to have larger facial muscles and require more toxin.
PHOTOGRAPHS
■ Static and dynamic (animated) preprocedure photographs are helpful, especially for patients not previously treated.
PATIENT EXPECTATIONS
■ Patients have varying desires for treatments. Some request near-complete denervation, whereas others prefer a more limited treatment.
■ Static lines frequently soften, but they are rarely eliminated. Treatment improves dynamic rhytids.
■ Longevity is approximately 3 months on average, but varies by dose, muscle bulk, and injection site.
TIP: In patients who have had prior treatments, a history including results and preferences is especially important.
INFORMED CONSENT
The risks, benefits, and alternatives to the procedure need to be presented. The planned injection sites may not be FDA-approved indications and are considered off-label use.
■ Recommended items to be included in the informed consent:
• A general description of the procedure and location of injections
• A sufficient description of potential risks
► Pain at injection site
► Bruising
► Eyelid or brow ptosis
► Headache
► Allergic reaction
► Nausea
► Facial asymmetry
► Dysphagia
► Respiratory compromise
PREPARATION AND EQUIPMENT2,13
RECONSTITUTION
■ The powder is usually reconstituted gently with 0.9% sterile saline solution.
■ When reconstituted, the solution should be clear, colorless, and free from particulate matter.
SENIOR AUTHOR TIP: Studies have shown that injection site discomfort is less when using preserved saline.14,15
STORAGE
■ Before reconstitution, unopened vials should be stored at 2°-8° C.
• Xeomin can be stored on the shelf at room temperature.
■ The manufacturer recommends using the toxin within 4 hours of reconstitution and storing it at 2°-8° C.
■ Literature indicates efficacy is maintained for up to 6 weeks with proper storage.
SYRINGE AND NEEDLES
■ 1 ml syringes allow injection of a precise volume of solution.
• Tuberculin or insulin syringes are most frequently used.
■ 30-gauge or 32-gauge needles
• Ultrafine needles limit injection site pain and allow tactile perception of muscular penetration.
SENIOR AUTHOR TIP: When performing multiple injections, changing needles after each syringe use is recommended. The fine tips dull quickly.
ANESTHESIA
■ Use of ice or cold packs, topical local anesthetic combinations, and other modalities can limit procedural discomfort.
■ This is not required, and use varies by physician and patient.
IMMUNOGENICITY11
■ Neurotoxins are macromolecular protein complexes with molecular weights of 300-900 kDa comprising a 150 kDa neurotoxin protein and varying amounts of nontoxin proteins.
■ The immune system may recognize any component of the protein complex and initiate an immune reaction, decreasing the effect of botulinum toxin over time.
■ Xeomin lacks a 900 kDa complexing protein. It has been postulated that this may result in a less immunosuppressant response compared with Botox and Dysport.
TECHNIQUE13
■ Treatment must be individualized based on muscle strength, skin thickness, degree of rhytids, choice of neurotoxin, and previous treatments.
■ Recommended doses are based on administration of Botox and Xeomin. Recommended ratio of 1:2 or 1:2.5 pertains to Dysport.
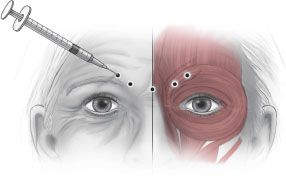
GLABELLAR COMPLEX
Vertical frown lines are the result of contraction of the muscles within the glabellar complex.
Anatomy
■ The corrugator supercilii, procerus, and depressor supercilii (medial orbicularis oculi) are the muscles responsible for brow depression.
• Corrugator supercilii muscles are composed of a transverse (larger) and an oblique head.
► Origin: Frontal bone at the superomedial orbital rim
► Insertion: Dermis at the middle third of the eyebrow, interdigitating with orbicularis occuli and frontalis muscle fibers
► Action: Depresses and adducts the eyebrows
► Dynamic rhytids: Vertical glabellar lines
• The procerus muscle is a flat, pyramidal muscle present on the bridge of the nose.
► Origin: Inferior aspect of the nasal bone and upper lateral nasal cartilages
► Insertion: Dermis overlying the nasal root
► Action: Depresses the medial brow
► Dynamic rhytids: Transverse lines on the nasal dorsum
• The depressor supercilii muscle is a portion of the medial orbicularis oculi, just inferior to the medial brow.
► Vertically oriented and just superficial to the corrugators at the superomedial orbital rim
► Action: Brow depression
► Dynamic rhytids: Contributes to both vertical and transverse glabellar lines and a lower static medial brow position
Injection
■ Five to seven injection sites are recommended (Fig. 21-1).