6 Basic science
Xeomin®
Summary and Key Features
• IncobotulinumtoxinA (NT 201, Xeomin®, Bocouture®) is an effective and well-tolerated treatment for facial wrinkles and is approved for the treatment of glabellar frown lines in many countries
• IncobotulinumtoxinA differs from other commercially available botulinum toxin type A products as it is free from complexing proteins and other impurities
• Complexing proteins have no therapeutic effect and do not prevent the active neurotoxin from diffusing and may lead to an increased risk of antibody formation
• IncobotulinumtoxinA is highly stable and can be stored at room temperature
• IncobotulinumtoxinA has a higher specific biological activity than other available botulinum toxin type A preparations, such as onabotulinumtoxinA (Botox® / Vistabel) or abobotulinumtoxinA (Dysport® / Azzalure®)
• In practice, the clinical equipotency of incobotulinumtoxinA and onabotulinumtoxinA has been proven when a dose conversion ratio of 1 : 1 is used. Multiple small dose injections result in a precise and predictable outcome
• The best results are achieved when the face is treated as a whole, since treating some muscles or areas in isolation can draw attention to the untreated areas
Properties of incobotulinumtoxinA
Purity
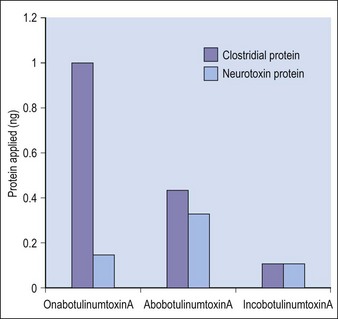
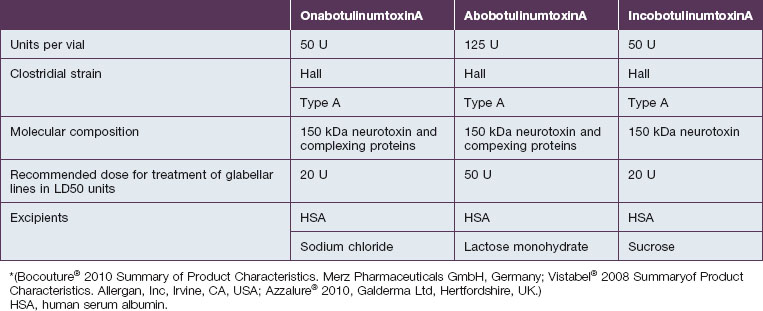
Botulinum toxin is produced by anaerobic fermentation of the bacterium Clostridium botulinum type A (Hall strain). During the manufacture of incobotulinumtoxinA, following fermentation and extraction of the toxin, the complexing proteins are removed by chromatography. Thus incobotulinumtoxinA contains markedly less clostridial protein than do onabotulinumtoxinA and abobotulinumtoxinA (Fig. 6.1). In addition, incobotulinumtoxinA has the highest specific biological activity of all three products. A high-sensitivity sandwich ELISA was used in a study by Frevert to measure the amount of botulinum toxin type A per 100 U of onabotulinumtoxinA, incobotulinumtoxinA and abobotulinumtoxinA. The results were 0.73 ng, 0.44 ng and 0.65 ng, respectively, giving incobotulinumtoxinA the highest specific biological activity (U/ng neurotoxin) at 227 U/ng compared with 137 U/ng for onabotulinumtoxinA and 154 U/ng for abobotulinumtoxinA (but it must be noted that the units of abobotulinumtoxinA are different from those of onabotulinumtoxinA and incobotulinumtoxinA). This suggests that, in addition to containing complexing proteins, onabotulinumtoxinA may also contain denatured / inactivated neurotoxin, unlike incobotulinumtoxinA (see Fig. 6.1). Like the other botulinum toxin type A products, incobotulinumtoxinA contains human serum albumin as an excipient but, whereas incobotulinumtoxinA contains sucrose, abobotulinumtoxinA contains lactose, and onabotulinumtoxinA contains neither of these sugars (Table 6.1).