“ So THAT’S how that happens! ”
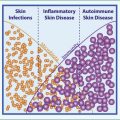
From birth, maybe even earlier, we learn how to interact with our environment. Our immune systems react fiercely to organisms like Staphylococcus aureus , yet develop tolerance to commensals like Staphylococcus epidermidis . Tiffany Scharschmidt (University of California, San Francisco) has uncovered some of the secrets of how and when our immune systems define friend versus foe, and the multiple dimensions involved.
Most warts resolve, sometimes spontaneously, while others do not. Why is one family member severely affected? Is it the difference in the virus, host, or some other factor? Drs Dimitrova and Freeman (NIH) describe the recently identified inherited disorders of DOCK8 and DOCK2 deficiency, which help understand why some patients develop widespread, recalcitrant disease, and how the dermatologist can be key to the early diagnosis of these disorders.
A series of autoinflammatory disorders with profound dermatologic manifestations have recently been characterized, not only clinically but also with a detailed understanding of underlying genetic mutations and inflammatory pathways. This has led to some highly effective, specific therapies. Raphaela Goldbach-Mansky and her team (NIH) have been instrumental in leading this discovery. They show us how to identify these disorders and understand their diverse skin presentations.
Why doesn’t psoriatic skin become infected, even after surgery, compared with other inflammatory skin conditions, like eczema? Why is atopic dermatitis skin prone to colonization and infection with S aureus ? Why is rosacea flared by sun—could it be related to vitamin D? Richard Gallo and his team (University of California, San Diego) have led the discovery of antimicrobial peptides in skin and their relationship to a spectrum of diseases.
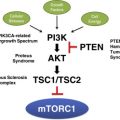
Early on, I was taught that every somatic cell in my body had identical genetic makeup. With the understanding that premalignant cells, such as keratinocytes, harbor DNA damage, it became clear that genetic mosaicism is widespread. Each of us is a mosaic. Depending on when in development the mosaicism occurs and which cell lineages are involved results in varied clinical presentation. Certain disorders can help us understand mosaicism, just as understanding mosaicism can help us understand disease pathogenesis. Tom Darling’s team (Uniformed Services University and NIH) studies somatic mutations in the mTORC1 signaling pathway, and they explain how mosaicism affects clinical presentation and implications for therapy.
While cylindromas were described over 150 years ago, it was the discovery of underlying CYLD mutations that demonstrated the link between several clinical syndromes. Neil Rajan and his team (Newcastle University, UK) describe this relationship and how understanding the pathophysiology is paving the way toward novel medical interventions for these disfiguring tumors.
Posttransplantation skin cancer is a major cause of morbidity and mortality. Why do patients fare worse on cyclosporine compared with other agents, such as sirolimus. John Carucci and his team (NYU) dissect the complex relationship between cyclosporine and interleukin-22 to uncover the interaction between the immune system, immunosuppression, and skin cancer and suggest new avenues to explore to harness this difficult management problem.
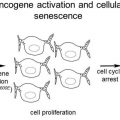
Approximately half of melanomas carry a mutation in the gene that encodes BRAF, a proto-oncogene involved in sending growth signals from outside cells to the nucleus. It is part of the RAS/MAPK pathway. Vemurafenib and dabrafenib, which target the BRAF V600E mutation, have been approved for metastatic melanoma. It is puzzling then, why this melanoma-associated mutation should be commonly present in benign nevi. Tom Hornyak’s research team (University of Maryland/Baltimore VA) describes how oncogene-induced senescence may explain the paradox.
The physical, cosmetic, financial, and psychological burden from scars induced by burns, trauma, surgery, and combat is daunting. Re-creating a more normal-appearing skin, with reticular and papillary dermis housing adnexal structures like hair follicles and sweat glands, could mitigate this morbidity. David Woodley (UCLA) describes newly identified features of fibroblasts that distinguish between different types of dermis, suggesting the first steps in the pathway from amorphous scar to regeneration of normal-appearing skin.
I hope you enjoy how these researchers are answering our clinical questions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree