4 Basic science
BOTOX® Cosmetic
Summary and Key Features
• The introduction of BOTOX® Cosmetic (onabotulinumtoxinA) into aesthetic dermatology has revolutionized the management of facial lines
• Botulinum neurotoxins are biological products, synthesized by bacteria, then purified, formulated, and packaged into minute quantities for medical use
• Seven different serotypes of botulinum toxins occur in nature (types A through G), although most clinical products, including onabotulinumtoxinA, are based on the A serotype
• Botulinum toxin type A has a well-defined mechanism of action; at the neuromuscular junction, it reduces acetylcholine release from motor nerves and inhibits muscular contractions
• Clinical and preclinical data suggest that onabotulinumtoxinA may also act on nociceptive neurons
• Each commercially available botulinum toxin product is a unique biological therapeutic, with a distinct structure, formulation, unit strength and clinical profile
• As biologics, the doses of botulinum neurotoxins are expressed in units of biological activity that are not interchangeable or convertible among different products
• Recent analyses demonstrate that the onset of effect of onabotulinumtoxinA in the management of glabellar lines occurs within 24 hours and that benefits last at least 4 months
• Although all botulinum toxin products may stimulate antibody formation, the immunogenicity profile of onabotulinumtoxinA is well characterized
• The clinical efficacy and safety profile of onabotulinumtoxinA in facial lines are well understood by skilled practitioners
Introduction
The introduction of botulinum toxin type A into the field of aesthetic dermatology has profoundly impacted the clinical management of undesirable facial lines. Botulinum toxins are injected into discrete facial muscles where they act locally to reduce muscle contractions that produce skin creases, either with facial animation or at rest. The pattern of injections can be tailored to individual needs and the results in glabella have been demonstrated by Carruthers and colleagues in 2004 to last an average of 4 months.
Serotypes and structure
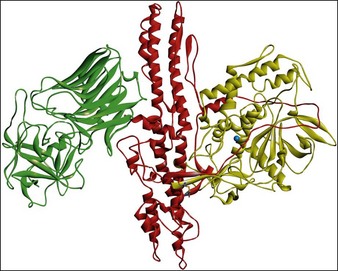
All botulinum toxins are produced by bacteria as protein complexes consisting of a core neurotoxin molecule with a molecular mass of approximately 150 kDa and one or more associated proteins. This protein contains three distinct functional domains. The binding domain is responsible for the docking of the molecule with its specific cell surface receptors, the translocation domain is critical in allowing the catalytic domain to access the neuronal cytosol, and the catalytic domain is responsible for the enzymatic activity that ultimately interferes with neurotransmitter release (Fig. 4.1).
Mechanism of action
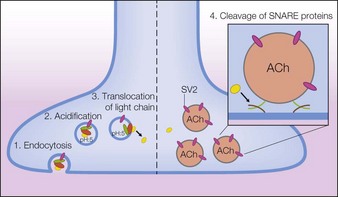
Following intramuscular injection, botulinum neurotoxins inhibit the release of acetylcholine (ACh) from motor nerve terminals, resulting in reduced muscular contractions. This inhibition occurs in multiple steps referred to as binding, internalization, translocation, and cleavage. Through a similar process, botulinum neurotoxins also inhibit acetylcholine release from autonomic nerve terminals that innervate smooth muscle or glands. Further studies have found that botulinum toxin type A exerts selective effects on the nociceptive system. These actions are described in the following text, which for onabotulinumtoxinA begins with the dissociation of the 150 kDa neurotoxin from the NAPs (Fig. 4.2).