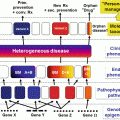
Disease
Mode of inheritance
Predominant ethnic group
Age at onset
Typical duration of flare
Typical frequency of flares
Typical/distinctive clinical features
Treatment
FMF
AR; rarely AD
Eastern Mediterranean
Childhood to early adulthood
1–3 days
Variable
Colchicine responsiveness
Colchicine
Pseudo-appendicular pain
Anakinra
Erysipelas-like erythema
TRAPS
AD
Northern European; numerous other ethnic groups
Childhood/early adulthood; rarely late onset
>7 days; may be prolonged over many weeks
Variable
Longer duration of attack; migratory myalgia with erythema; periorbital edema
Steroids on demand
Etanercept
MVK deficiency/HIDS
AR
Northern European
Infancy
3–7 days
1–2 monthly
Palpable lymph nodes; diarrhea; triggered by vaccinations
Steroids on demand
Anti-TNF
Anti-IL-1
FCAS
AD
Northern European
Childhood
24 h
Depends on exposure to cold
Triggered by exposure to cold
Cold avoidance
Neutrophilic urticarial dermatosis
Anti-IL-1
Conjunctivitis
Thirst and transpiration
Spectacular response to anakinra
MWS
AD
Northern European
Neonatal/infancy
Continuous; worse in the evening
Often daily
Neutrophilic urticarial dermatosis
Anti-IL-1
Hearing loss
Spectacular response to anakinra
CINCA
Sporadic
Northern European
Neonatal/infancy
Continuous
Continuous
Neutrophilic urticarial dermatosis
Anti-IL-1
Dysmorphia
Aseptic meningitis
Deforming arthropathy
Spectacular response to anakinra
PAPA
AD
Northern European
Childhood
Intermittent
Variable
Pathergy
Anti-TNF
Notion of familial pyoderma gangrenosum
Anti-IL-1
Migratory arthritis in early childhood
DIRA
AR
Newfoundland; Brazil; Lebanon; Puerto Rican, Dutch
Neonatal/infancy
Continuous
Continuous
Pustules
Anti-IL-1
Osteolytic bone lesions
Spectacular response to anakinra
CANDLE
AR
Japan
Infancy
Continuous
Continuous
Exacerbated in winter
No efficient treatment known
Plaques with atypical myeloid infiltrate
Pernio-like lesions
Lipoatrophy
Basal ganglia calcification
Blau syndrome
AD
Not known
Childhood
Continuous
Continuous
Granulomatous dermatitis
Steroids
Uveitis
Anti-TNF
An AIS should be suspected in every patient with otherwise unexplained recurrent flares of inflammation with or without fever. Age of onset, type of involvement, and duration of the attacks will help establishing a correct diagnosis (Table 7.1). In this author’s experience, a significant number of patients have however all the characteristics of a typical AIS, but they do not fit into established nosology, as the whole spectrum of these disorders is so far not delineated and new entities are regularly reported and mutations in new genes described.
We shall only briefly describe the cutaneous findings of some of these disorders, especially those that are clearly IL-1 mediated [4]. This will allow us to draw conclusions that we can apply to the much commoner complex polygenic disorders.
7.2.1 Clearly IL-1-Mediated AIS: IL-1 Is the Only or Main Pathogenic Factor
7.2.1.1 Excessive IL-1 Production: Cryopyrinopathies or Cold-Induced Autoinflammatory Syndromes (CAPS)
They include three, partially overlapping autosomal dominant entities related to mutations in the same NLRP3/CIAS1 gene: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and chronic infantile neurological and articular (CINCA)/neonatal onset multi-inflammatory disorder (NOMID) [3, 4]. Within the CAPS spectrum, FCAS is the least severe entity, while CINCA is the most severe.
A closely related syndrome to the milder FCAS/MWS variants was described in patients with a NLRP12 mutation [6].
The three disorders usually start in the newborn or during early infancy with fever, fatigue, a flu-like syndrome, and rash. The flares are triggered by exposure to cold in FCAS – after an interval of 1–2 h – and are accompanied by thirst, transpiration, joint pain, and conjunctivitis. Cold-stimulation tests (ice cube, immersion) can be negative, as ventilated cold is the usual trigger. Cold dependency of flares is less marked in MWS, but this disorder is more severe and patients develop sensorineural hearing loss and they are at risk of AA amyloidosis.
Continuous flares with neutrophilic aseptic meningitis, dysmorphism, mental retardation, sensorineural deafness, and deforming arthropathy characterize CINCA, a very severe disorder. Diagnosis is based on typical clinical and biological findings; evidencing an autosomal dominant mutation in NLRP3/CIAS1 gene is helpful, but the mutation will not be present in all patients.
Skin Findings and Histopathology
Neutrophilic urticarial dermatosis (NUD; see also below) is the classic cutaneous manifestations in those children [7, 8]. An urticarial rash with a neutrophilic intravascular, perivascular, perieccrine, and interstitial infiltrate on histopathologic evaluation is typical. In this author’s experience, leukocytoclasia is frequent in patients with NUD in the context of Schnitzler’s syndrome (see below) but much less so in children with CAPS.
7.2.1.2 Deficient IL-1 Inhibition: Deficiency of Interleukin-1 Receptor Antagonist (DIRA)
DIRA is suspected in every newborn with a pustular dermatitis, multifocal osteomyelitis, and periostitis with marked elevation of acute phase reactants though fever is only low grade or absent. Pustules are aseptic. Chronic lung disease, respiratory distress syndrome, and central nervous system vasculitis were rarely described. Fatal evolution is reported [9, 10]. Diagnosis relies on clinical and radiological findings (widened rips and clavicles, osteolytic lesions of long bones) and is supported by evidencing an autosomal recessive loss-of-function mutation in IL-1Rn gene [9].
Skin Findings
Grouped pustules on an erythematous base in the newborn or within the first 3 weeks and evolution toward yellowish crusts [9, 11]. The lesions can be localized or widespread, including face and scalp involvement. Bullae with hypopyon can be present. Evolution toward ichthyosiform lesions with diffuse desquamating scaly and sometimes slightly red skin can occur [12]. Oral mucosa and nail can be affected, with pitting and onychomadesis.
Histopathology
Epidermal spongiosis and acanthosis and most notably a neutrophilic infiltrate of the epidermis, with intracorneal, subcorneal, and intraepidermal microabscesses, as well as a neutrophilic infiltrate in the dermis and the perifollicular and perieccrine areas [9, 11]. Neutrophilic syringotropism could be a distinctive feature [12], and this latter finding is also found in CINCA syndrome [8].
7.2.2 AIS in Which IL-1 Plays an Important Role, but Other Factors Are Involved
7.2.2.1 Familial Mediterranean Fever (FMF)
The disease usually starts before the age of thirty with recurrent flares of fever; abdominal pain, sometimes mimicking an acute abdomen; pleurisy; and large joint arthritis that last between 1 and 3 days. The major risk is the development of inflammatory AA amyloidosis, and this risk can be largely prevented with continuous treatment with colchicine. Diagnosis is supported by evidencing a pathogenic autosomal recessive MEFV gene mutation (rare dominant variant exist); diagnosis is based on the Livneh criteria [13].
Typical Skin Findings
The most typical cutaneous finding is the so-called erysipelas-like erythema [14, 15]. It consists of a red edematous, warm, swollen erythema, more often than a circumscribed plaque. The erythema is usually localized on the lower limbs below the knee, typically in the perimalleolar area or the dorsum of the foot.
7.2.2.2 Pyogenic Sterile Arthritis, Pyoderma Gangrenosum, and Acne (PAPA) Syndrome
Early-onset childhood flares of recurrent painful sterile arthritis, sterile abscesses, and pathergy are typical. By puberty, joint symptoms tend to subside, while skin symptoms increase. Diagnosis is established on grounds of clinical history and finding an autosomal dominant mutation in PSTPIP1 [16].
Skin Findings
Pathergy and aseptic abscesses, as well as ulcerations related to pyoderma gangrenosum, can occur from childhood on. Gingival pustules can also occur from childhood on [16]. By puberty, severe nodulocystic acne develops. PAPA should be considered in every patient with a familial history of pathergy and/or pyoderma gangrenosum.
Hidradenitis suppurativa can also develop and has been reported in a patient with a novel PSTPIP1 mutation; authors then called this expanded entity PAPASH [17]. A related entity referred to as “PASH syndrome” has been described [18]. These patients lack the sterile arthritis, but they have nodulocystic acne and pyoderma gangrenosum; in addition, they develop hidradenitis suppurativa. The underlying genetic abnormality is so far unknown.
Histopathology
There are no specific histopathological findings reported so far.
7.3 Pathophysiology
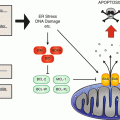
CAPS are related to mostly missense mutations in the NLRP3/CIAS1 gene encoding a death domain protein known as NLRP3 (or cryopyrin). This protein is expressed in the epithelial cells of the skin and the mucosa, the granulocytes, the dendritic cells, and the T and B cells. A variety of danger signals, including “pathogen-associated molecular pattern” (PAMP), induce association of NLRP3 with other members of the death domain superfamily to form a cytosolic protein complex named the “inflammasome.” This results in activation of caspase 1 which cleaves biologic inactive pro-IL 1β into biologic active IL-1β [3, 4]. It also upregulates NF-κB expression and thereby increases IL-1 gene expression. IL-1 is a major proinflammatory cytokine and the key mediator of the manifestations of CAPS. This assumption is supported by the observation that IL-1 blockade induces rapid and complete response in patients with CAPS.
DIRA is related to a deficiency of the naturally occurring antagonist of the IL-1 receptor (IL-1Rn) [9, 11]. The result is an excess in IL-1-mediated inflammation, by lack of inhibition of (initially) normally produced IL-1.
The pathophysiology of FMF is less clear. FMF is usually an autosomal recessive disorder related to mutations in the MEFV gene, though rare dominant mutations exist [19]. MEFV encodes pyrin, which plays probably an important role in the modulation of caspase 1 and thus the production of IL-1.
Mutations in PSTPIP1




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree