Fig. 24.1
Structures and nomenclature of ceramides (CER) in human stratum corneum (SC). (Note: this research was originally published in J. Lipid Res. (Masukawa et al. 2008). © the American Society for Biochemistry and Molecular Biology)
Table 24.1 shows a summary of intercellular lipids reported in the SC of AD lesional, AD nonlesional and controlled healthy nonlesional skin. Although numerous studies have emphasized diverse results due to the different subjects tested and the different methods used, there are common features for AD lesional skin as follows: (1) the level and/or wt. % of total CER is lower; (2) the CER composition is altered; and (3) the chain length of CER species is shortened. The first feature was confirmed by analyses done by Imokawa et al. 1991; Matsumoto et al. 1999; and Ishikawa et al. 2010. The second feature, i.e., that the balance of CER[EOS], other EO-containing CER subclasses and CER[NP] is commonly altered, was reported by Imokawa et al. (1991); Di Nordo et al. (1998); Matsumoto et al. (1999); Ishikawa et al. (2010); and Angelova-Fischer et al. (2011). The third feature, most recently unveiled, comes from the significantly higher levels of CER[NS], CER[NDS], and CER[AS] with shorter chain lengths, as represented in C34-CER[NS] (Ishikawa et al. 2010). The validity of the third feature is corroborated by the fact that there were significantly lower levels of CER[NS], CER[NDS], CER[NH], CER[AS], and CER[AH] with longer chain lengths in the AD lesions (Ishikawa et al. 2010), the fact that a CER[AS] species with a shorter chain length was detected in AD nonlesional skin but not in healthy skin (Bleck et al. 1999), and the fact that significantly higher wt. % of C34-CER[NS], C-34CER[NH], C34-CER[AS], and C-34CER[AH] were found in AD nonlesional skin (Janssens et al. 2012). Macheleidt et al. (2002) found a lower wt. % of very-long-chain FFA in the SC of AD lesional skin although this is not for CER.
Table 24.1
Skin lipids for AD lesional (AL), AD nonlesional (ANL), and controlled healthy nonlesional (HNL)
Authors | Materials | Methods | Results |
|---|---|---|---|
Melnik et al. 1988 | SC from 10 ANL and 10 HNL | TLC | Lower wt. % of total CER in ANL |
Imokawa et al. 1991 | Cyanoacrylate-stripped SC from 35 AL, 35 ANL, and 65 HNL | TCL | Lower level of total CER in AL and ANL |
Lower wt. % of CER 1 (CER[EOS]) in AL and ANL | |||
Yamamoto et al. 1991 | Extracted SC lipids from 6 ANL and 6 HNL | TLC | Lower wt. % of CER 1 (CER[EOS]) in ANL |
Di Nardo et al. 1998 | Cyanoacrylate-stripped SC from 28 AL, 19 ANL, and 20 HNL | TLC | Lower levels of CER 1 (CER[EOS]) and CER 3 (CER[NP]) in AL |
Higher wt. % of CH in AL and ANL | |||
Intermediated levels in ANL between AL and HNL | |||
Matsumoto et al. 1999 | Extracted SC lipids from 14 AL,30 ANL, and 25 HNL | TLC | Lower levels of total CER and CER 1 (CER[EOS]) in AL |
Not different between ANL and HNL | |||
Bleck et al. 1999 | Cyanoacrylate-stripped SC from 10 ANL and 10 HNL | TLC | Lower wt. % of CER[EOS] and CER[NP], higher wt% of CER[EOP], and shorter chain in CER[AS] in ANL |
MALDI-MS | |||
Macheleidt et al. 2002 | Biopsied epidermis from 10 AL, 8 ANL, and 5 HNL | TLC | Lower wt. % of ω-hydroxy CER in AL and ANL |
GC | Lower wt. % of very-long-chain FFA in AL and ANL | ||
Arikawa et al. 2002 | Tape-stripped SC from 73 AL, 83 ANL, and 69 HNL | TLC | Lower level of sphingosine in AL and ANL |
Okamoto et al. 2003 | Tape-stripped SC from 44 AL, 47 ANL, and 40 HNL | TLC | Lower level of sphingosylphosphorylcholine in AL and ANL |
Ishibashi et al. 2003 | Tape-stripped SC from 92 AL, 105 ANL, and 81 HNL | TLC | Lower level of glucosphingosine in AL and ANL |
Farwanah et al. 2005b | Extracted SC lipids from 7 ANL and 7 HNL | TLC | Not different in CER between ANL and HNL |
LC-MS | |||
Ishikawa et al. 2010 | Tape-stipped SC from 7 AL, 7 ANL, and 7 HNL | LC-MS | Lower levels of total CER, CER[NH], CER[NP], CER[EOS], CER[EOH], and CER[EOP], higher level of CER[AS], lower levels of longer chain in CER[NS], CER[NDS], CER[NH], CER[AS], and CER[AH], and higher levels of shorter chain in CER[NS] (especially with C34), CER[NDS] and CER[AS] in AL |
Intermediated level in ANL between AL and HNL | |||
Jungersted el al. 2010 | Cyanoacrylate-stripped SC from 12 ANL-FLGm, 19 ANL-FLGw, 6 HNL-FLGm, and 12 HNL-FLGw | TLC | Lower wt. % of CER[EOP] and higher wt. % of CER[AP] in ANL-FLGm than HNL-FLGm and HNL-FLGw |
Lower wt. % of CER[EOS] and CER[AP] in ANL-FLGw than HNL-FLGm and HNL-FLGw | |||
Angelova-Fischer et al. 2011 | Cyanoacrylate-stripped SC from 14 AL/ANL-FLGm, 23 AL/ANL-FLGw, and 20 HNL | TLC | Lower levels of CER[EOH] and fatty acids in AL-FLGm than AL-FLGw |
Higher level of CH in ANL-FLGm | |||
Janssens et al. 2011 | Tape-stripped SC from 6 ANL and 6 HNL | LC-MS | Lower wt. % of CER[NP] and (CER[EODS] + CER[EOS] + CER[EOP] + CER[EOH]) in ANL |
Janssens et al. 2012 | Tape-stripped SC from 14 ANL-FLGm, 14 ANL-FLGw, and 15 HNL | LC-MS | Higher wt. % of shorter C34-CER[NS], C34-CER[NH], C34-CER[AS], and C34-CER[AH] and lower wt. % of (CER[EODS] + CER[EOS] + CER[EOP] + CER[EOH]) in ANL with no differences between FLGm and FLGw |
Compared with the lipid abnormalities in AD lesional skin, AD nonlesional skin looks somewhat indefinite in terms of the levels and composition of CER. As shown in Table 24.1, the intermediate features of AD nonlesional skin between AD lesional skin and healthy skin were described in some articles (Di Nardo et al. 1998; Ishikawa et al. 2010) and characteristics in the lipid abnormalities similar to AD lesions were shown in AD nonlesional skin by others (Bleck et al. 1999; Janssens et al. 2011, 2012). On the other hand, Matsumoto et al. (1999) and Farwanah et al. (2005b) reported no differences between AD nonlesional skin and healthy skin. Those inconsistencies in results obtained for nonlesional SC of AD skin would be due to the varieties of subjects tested (severity, progress, and degree of nonlesions), sampling sites/procedures and analytical methods used. Filaggrin gene mutations do not appear to directly influence the lipid abnormalities for the nonlesional SC of AD skin. No significant differences at the nonlesional sites were found between individuals carrying and not carrying the mutations (Jungersted et al. 2010). In another study undertaken by Janssens et al. (2012), the nonlesional SC of AD subjects carrying filaggrin mutations did not have any differences in lipids with those not carrying the mutations. However, the lower level of CER[EOH] in the lesional SC of AD patients carrying the mutations than those not carrying them was pointed out (Angelova-Fischer et al. 2011). To define characteristics of the lipids in AD nonlesional skin and the impact of filaggrin gene mutations on the lipids in the SC of AD skin, much larger-scaled studies are needed.
Collectively, the answer for the question “are SC lipids in AD skin different from the lipids found in normal skin?” is likely “yes” for the SC of AD lesional skin, as indicated by the lower level of total CER, the altered CER composition and the CER species with shorter chain lengths. For AD nonlesional skin, the abnormalities may be present with slight but similar characteristics to AD lesional skin, but further studies are required in a way that the subjects tested are standardized in terms of severity, progress, and degree of nonlesional skin. The filaggrin gene mutations do not seem to directly affect the lipid abnormalities, at least for the nonlesional SC of AD skin but this also remains to be defined.
Do the Lipid Abnormalities Affect the Structures and/or Properties of Skin?
No studies have been reported that characterized structures of the lipid bilayer at intercellular spaces in the SC of AD lesional skin, and only structures in nonlesional SC have been investigated. The long-periodicity phase in the lipid bilayer in the nonlesional SC was found to be slightly but significantly reduced in the repeat distance or repeat quantity compared to healthy SC (Janssens et al. 2012). Regarding the lateral lipid packing, it was found that the nonlesional SC of AD patients has an increased percentage of hexagonal lattice, gel phase, compared to healthy skin which is characterized by a larger presence of orthorhombic packing, crystalline phase (Pilgram et al. 2001; Janssens et al. 2012, 2013). These differences could be interpreted as originating from the lipid abnormalities, such as a lower level of total CER, an altered CER composition, and/or CER species with shorter chain lengths.
The diminished level of total CER in the SC of AD skin had a negative correlation with transepidermal water loss (TEWL), which is an index of impaired barrier function (Ishikawa et al. 2010). Also, there was a significantly negative correlation of the TEWL value versus the level of each CER subclass other than CER[AS] and CER[NS]. The subclass of CER[AS] had a significantly positive correlation with TEWL (Ishikawa et al. 2010). Only the subclass CER[AS] seems to have a different nature in terms of the involvement with the barrier function in AD skin. An effect of chain lengths of CER species on the TEWL has also been revealed. Thus, the more abundant the CER species with shorter chain lengths are, the higher the TEWL values (Ishikawa et al. 2010; Joo et al. 2010; Janssens et al. 2012). Since the level of C34 CER species sounds strongly correlated with TEWL (Ishikawa et al. 2010; Janssens et al. 2012), it may be a characteristic marker for the diagnosis of AD. Janssens et al. (2013) showed that the change in tendency in the lateral packing is correlated with the TEWL value. That correlation could be interpreted by the physicochemical nature that the hexagonal lattice is a less-packed structure in the lateral direction, where water can be less disturbed through the lipid bilayer.
The structure of the lipid bilayer in the SC of AD skin is likely to be changed into a bilayer with the reduced repeat distance or repeat quantity in the long-periodicity phase and with an increase in the hexagonal lattice, which may be due to the lipid abnormalities. This change in structure would cause a higher TEWL value corresponding to the impaired barrier function of AD skin.
Is the Mechanism Underlying the Lipid Abnormalities Known?
An ultrastructural study of AD skin versus healthy skin indicated the immature formation of lipid lamellae at the border between the stratum granulosum and the SC of AD skin (Fartasch et al. 1992). Thus, in AD skin, lamellar body-discs remained undelivered and were found even within the horny cells, in contrast to healthy skin where the body-discs completely disappeared. This suggested an abnormal keratinization coming from the unusual lipid metabolism in AD skin. The deficiency of CER in the SC of patients with AD can be explained by the extraordinary upregulation of glucosylceramide sphingomyelin deacylase (GSDase), which hydrolyzes glucosylceramide (GlcCER) or sphingomyelin (SM) at an acyl site to yield sphingosylphosphorylcholine (SPC) or glucosylsphingosine (GSP), respectively, instead of CER (Imokawa 2009), as illustrated in Reaction 1 of Fig. 24.2. The substantiality of the enzyme is supposed to be the β-subunit of acid ceramidase (CDase) based on a study using rat skin (Nogami-Itoh et al. 2010). At first, it was found that in the skin of patients with AD, the activities of three sphingolipid hydrolysis enzymes, β-glucocerebrosidase, sphingomyelinase (SMase), and CDase were not changed (Jin et al. 1994; Murata et al. 1996) whereas SM hydrolysis was increased with the occurrence of SPC as a reaction product and this hitherto undiscovered enzyme was tentatively termed SM deacylase (Murata et al. 1996; Hara et al. 2000). In a subsequent study, this enzyme was then termed GSDase because it hydrolyzes not only SM but also GlcCER in AD skin (Higuchi et al. 2000). The fact that the levels of SPC and GSP were both significantly higher in the epidermis of AD patients (Okamoto et al. 2003; Ishibashi et al. 2003), as listed in Table 24.1, corroborates the mechanism that the upregulation of GSDase generates the CER deficiency.
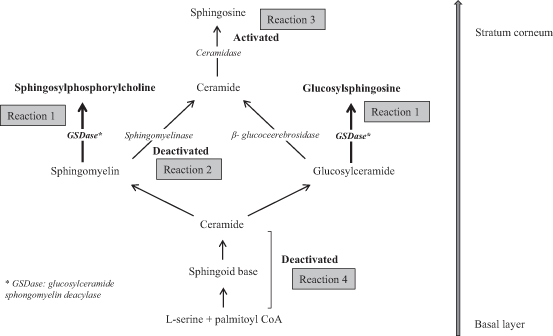
Fig. 24.2
Metabolism of ceramides (CER) in human skin
Other possible mechanisms underlying the diminished level of CER were proposed regarding reduced SMase activity (Reaction 2 of Fig. 24.2) and the involvement of bacterial CDase (Reaction 3 of Fig. 24.2). Acid SMase as well as neutral SMase, which produce CER from SM in the epidermis, were decreased both in the lesional and nonlesional skin from AD patients compared to control healthy skin (Jensen et al 2014). The involvement of bacteria secreting CDase by which CER would be decomposed in the SC of AD skin was proposed (Ohnishi et al. 1999). Those mechanisms might be responsible in part for the diminished level of total CER. However, the altered CER composition cannot be explained only by the SMase activity or bacterial CDase because CER[NS] and CER[AS] are derived in part from the corresponding SM precursors while other subclasses such as EO-containing CER are derived only from GlcCER (Uchida et al. 2000; Hamanaka et al. 2002). Those mechanisms are not enough to explain the altered CER composition in the selective changes in the balances of CER[EOS], other EO-containing CER subclasses, and CER[NP].
Macheleidt et al. (2002) compared the de novo synthesis of GlcCER and CER in lesional AD skin with healthy skin using a metabolic labeling technique, which revealed remarkable decreases of newly biosynthesized ClcCER and CER in lesional AD skin. An experimental system using a reconstructed human epidermal keratinization model suggested that the Th2 type of inflammation evoked in AD skin may be one factor involved in the downregulated biosynthesis of CER, which results in the reduced levels of CER in the SC (Sawada et al. 2012). Therefore, the deficiency of CER in the SC of AD skin is likely to be caused not only by the abnormal pathway from GlcCER and SM to CER, such as the upregulation of GSDase (Reaction 1 of Fig. 24.2), but also reduced the de novo synthesis of CER skeletons themselves (Reaction 4 of Fig. 24.2). As for the chain length, elongases in the epidermis seem to be involved. Although the results were obtained in an experimental system using mice but not humans, some elongases that synthesize very-long-chain FFA were downregulated in a hapten-induced AD model (Park et al. 2012). It could be assumed that the downregulated elongases resulted in the decreased levels of CER species with longer -chain lengths (Bleck et al. 1999; Ishikawa al. 2010; Janssens et al. 2012)very-long-chain FFA (Macheleidt et al. 2002).
Based on evidence accumulated to date, the mechanism underlying the lipid abnormalities for the SC in AD skin can most probably be explained by a combination of events, as follows: (1) the lower level of total CER would be caused by both the upregulation of GSDase and the reduced de novo synthesis of CER in the epidermis of AD skin, (2) the altered CER composition might be ascribed to changes in activities of enzymes relevant to the production of CER in the SC although this remains to be clarified, and (3) CER species with shorter chain lengths might originate from downregulated elongases although that also remains to be elucidated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






