11 Aquamid®
Summary and Key Features
• Aquamid® is a homogeneous polyacrylamide hydrogel (PAAG) containing no microparticles that is used as a tissue filler with a long-lasting effect
• PAAG belongs to the most commonly used permanent fillers worldwide
• It is best used for facial volumizing and deep structural augmentation
• Effective treatment must be performed using aseptic technique with injections subcutaneously into the deep layers of tissue in a retrograde fan-like manner
• PAAG has an immediate filling effect, adding volume; it does not depend on foreign-body reaction
• Aquamid® has been shown to have favorable physical–chemical interaction between PAAG and surrounding tissue, being accessible to water and solutes, including benzyl penicillin
Product
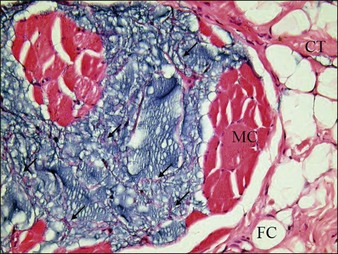
Studies by Bello and colleagues and by De Cassias Novaes & Berg have shown that this PAAG does not migrate within the tissue after subcutaneous injection because of its large molecular size and high cohesive properties. Christensen found that cells grew well in the Aquamid® gel, which allows vessel ingrowth from adjacent tissue. It is kept in place by means of a fibrous capsule (endoprosthesis) that develops after being injected in the subcutaneous tissue. These capsules are surrounded by fibroblasts and macrophages (Fig. 11.1). Another study by Zarini and colleagues emphasized the importance of the microstructure and porosity of hydrogels in relation to their functions and interactions with surrounding medium and tissue. Hydrogels could behave as an accessible foreign body that creates the appearance of infections that are untreatable. PAAG can exchange both physiological and non-physiological constituents very efficiently with the surrounding medium. In the context of hydrogels as tissue fillers, Brahm et al considered the ability of water and solutes (including an antibiotic) to cross between the hydrogel and the surrounding tissue to be attractive.