Fig. 33.1
Mechanism of action of bulking agents. (a) Injection in the internal anal sphincter defect. (b) Injection by quadrants
Types of Agents
The ideal filling material should be noncompatible and nonimmunogenic and it should induce a minimum inflammatory and fibrotic response [14]. The agent particles should be big enough to avoid migration out of the injection site (a diameter greater than 80 μm), but they should also be durable enough to last. Animal studies have shown that there is distant migration of particles with diameters of 4–80 μm, with particles found in lymph nodes and the lungs, kidneys, spleen, and brain [15]. With migration there is poor durability and, more seriously, the possibility of formation of a chronic granuloma at the site of migration. In general, most current materials consist of particles suspended in an excipient (an inactive carrier), which is usually a biodegradable gel.
Characteristics of the ideal agent include the following:
Biocompatible
Nonmigratory
Nonallergenic
Nonimmunogenic
Noncarcinogenic
Easy to inject
Produces durable results
The carcinogenic potential of implanted prosthetic materials has been examined in animals, but it has yet to be established in humans [16, 17].
Polytetrafluorethylene
The injection of polytetrafluoroethylene (Polytef or Teflon; DuPont, Wilmington, DE) was first reported by a urologist approximately 20 years before Shafik’s [18] report of its use in fecal incontinence, with one study started by Politano [19] as far back as 1964. Polytetrafluoroethylene is produced by the pyrolysis (a thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen) of Teflon. Polytef particles range in size from 4 to 100 μm, with 90 % in the 4 to 40 μm range. The filling material used in the injection treatment is a paste consisting of polytetrafluoroethylene, glycerin, and polysorbide [20]. The clinical use of this product has been interrupted because of the real possibility of migration [21].
Autologous Fat
The use of an agent extracted from the patient is an attractive option with respect to the reduction of inherent immunogenicity. Cells are extracted from the fat of the abdominal wall by suction. They subsequently are purified and suspended again in saline solution to be injected into the anal canal. The rapid digestion and migration potential of this material, however, has stopped the further development of this option [22], although Shafik [23] had initially published a report of its successful use in the treatment of fecal incontinence. The only report of a death after periurethral injection of a bulking agent involved injection of autologous fat in a urological setting [24], where pulmonary adipose tissue and lipid droplet embolism was found during postmortem examination.
Silicone Particles
Silicone is the volume augmentation agent most widely used to date in the treatment of fecal incontinence. The name Macroplastique is familiar to urologists, and it was this same substance that was reported as a bulking agent for fecal incontinence, although it was marketed under the name Bioplastique [25, 26]. It has since been renamed as PTP Implants and PTQ Implants (Uroplasty BV, Geleen, The Netherlands).
The PTQ Implants product is a heterogeneous injectable material consisting of polydimethylsiloxane particles suspended in a bioexcretable carrier, hydrogel of polyvinylpyrrolidone (povidone, PVP). The solid particle content represents approximately one third of the volume of the agent, and the particle size generally falls within the 100 to 450 μm range, but there are smaller particles within the gel [27]. Particle flexibility and texture enable collagen deposition in an irregular way around and through the implant. This all-around deposit prevents the implant, at least in theory, from being compressed and turned into a hardened paste. It also keeps it in place with a constant volume (Fig. 33.2).
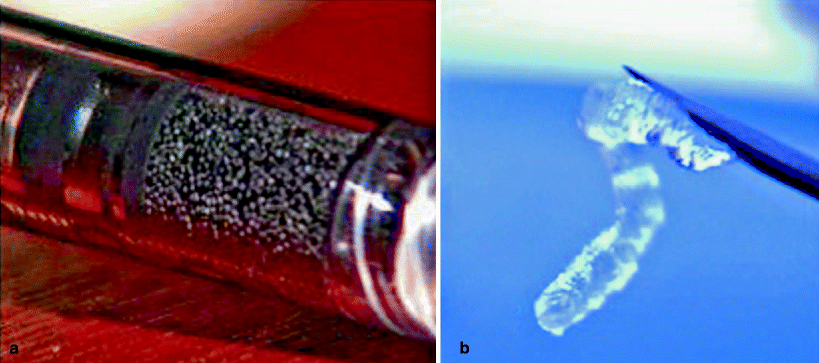

Fig. 33.2
Detailed aspects of two bulking agents: (a) PTQ Implants. (b) Durasphere
Animal studies have produced variable results depending upon particle size, and the potential for migration of smaller particles has been reported [28, 29], which raises the possibility of granuloma formation. In this regard, a study of rats injected with Bioplastique showed a minimal local reaction and lack of distal migration [30]. Recently, de la Portilla et al. [31] have proved through imaging that the silicone implants may move or even be lost; however, there is still doubt about whether the nonvisualization of the implants in some patients is due to a fragmentation of the implants into small particles that are not detected by endosonographic means. There have been further concerns about a possible link between silicone and autoimmune disease, but recent data seem to refute this [32, 33].
Bovine Collagen Treated with Glutaraldehyde Cross-Linked Collagen (GAX-Collagen)
Bovine collagen treated with glutaraldehyde (Contigen; Bard, Covington, GA) is formed from dermal bovine collagen cross-linked with glutaraldehyde and dispersed in a physiological saline solution saturated with phosphate [34]. At least 95 % of GAX-collagen is collagen type I and between 1 and 5 % is collagen type III [20]. There is no problem with granuloma formation or migration, and it is biocompatible and does not begin to degrade for approximately 12 weeks after the injection. The persistence of GAX collagen has been verified histologically 9–19 months after injection [35, 36]. An allergic response occurs in up to 3 % of patients, and subclinical seropositivity has been reported, although with no adverse effect [37]. Before injecting GAX-collagen, a cutaneous test must be performed to detect any hypersensitivity reaction.
Carbon Beads
This bulking agent (Durasphere, Carbon Medical Technologies, Inc., St. Paul, MN) consists of solid, pyrolytic, carbon-coated beads suspended in a viscous carrier gel. The carrier gel consists of water and beta-glucan. The carbon-coated beads are approximately three times larger than the migration threshold of 80 μm and cannot be absorbed [38]. Pyrolytic carbon is biologically nonreactive. There is some resistance to flow, and injection requires an 18-gauge needle. The beads are not biodegradable and therefore may have the advantage of durability over those agents that are prone to break down over time. In urological study, significant migration into the local and distant lymph nodes, as well as into the urethral mucosa, was noted [39] (see Fig. 33.2).
Hydroxyapatite Ceramic Microspheres
This bulking agent (Coaptite, Bioform, Franksville, WI) consists of hydroxyapatite ceramic microspheres suspended in a carrier gel of sodium carboxymethylcellulose, glycerin, and water. The particles are manufactured to be 75–125 μm in size to avoid migration. They are also nonantigenic and noninflammatory. After injection they become enmeshed within a nonencapsulated, stable, soft collagen matrix, which results in maintenance of volume even after the solid particles have been slowly degraded and resorbed. Two major advantages of its use include its ease of injection through a 21-gauge needle and its radiopacity, making it visible on plain films [40].
Dextranomer/Hyaluronic Acid Copolymer
This volume augmentation agent (Solesta, QMED, Uppsala, Sweden) consists of dextranomer microbeads and nonanimal, stabilized hyaluronic acid gel with a diameter of 120 μm [41]. The dextranomer facilitates the ingrowth of fibroblasts and the production of collagen between the microspheres as the hyaluronic acid is degraded so that the bolus is consolidated with endogenous tissue, stabilizing its volume for a sustained, long-term response. The biological evaluation of the product in gel in the treatment of fecal incontinence is based on biocompatibility tests performed with the final dextranomer and nonanimal, stabilized hyaluronic acid products.
The most important finding of preclinical studies has been that the product was well tolerated and has not shown any sign of cytotoxicity, teratogenicity, genetic destabilization, or general toxicity [42, 43]. Likewise, its biocompatibility in vivo has been shown to be good; for up to 2 years, the implant does not seem to migrate to other tissues [44].
Cross-Linked Porcine Dermal Collagen
This is a biological material (Permacol, Tissue Science Laboratories PLC, Aldershot, UK) containing large particles of cross-linked porcine dermal collagen; in its sheet form, it has been implanted in more than 75,000 patients in a variety of repair procedures over the last 6 years. Cross-linked porcine dermal collagen now is being introduced as an alternative biocompatible, nonallergenic collagen product with improved durability as a result of revascularization and cell ingrowth. It is relatively easy to inject [45].
Cross-Linked Polyacrylamide
This is a synthetic, nonparticulate hydrogel (Bulkamid, Contura International A/S, Soeborg, Denmark) consisting of 97.5 % water and 2.5 % cross-linked polyacrylamide. It is biocompatible but not biodegradable. It is nonresorbable, resistant to migration, and known to cause little reaction in the surrounding tissue. As a homogeneous hydrogel with no particles, it is said to retain elasticity and does not cause hard tissue fibrosis and, as a consequence, it is effectively nonallergenic [45].
Microballoons
Expandable microballoons (Urosurge Inc., Corallville, IA) are made of silicone and the filler consists of a biocompatible hydrogel made of poly-N-vinyl-pyrrolidone. The delivery system consists of a trocar-like needle made of stainless steel, a sheath, and the delivery catheter with the microballoon attached to its distal end. Experience with this bulking agent is limited to only one study; therefore, conclusions regarding its safety cannot effectively be drawn at present [46].
Stem Cells
In recent years, several studies have investigated the ability of mesenchymal stem cells (MSCs) to differentiate into mature cells of many tissues, both in vitro and in vivo, and specific improvement of tissue repair has been described in different organs after injury and stem cell injection. In muscular tissue, in particular, injected MSCs have the capacity to engraft and form multinucleated myotubes participating effectively in regeneration after injury [47]. The injection of stem cells as a potential treatment of urinary incontinence has been successfully reported in animal models. Injected stem cells have been shown to enhance regeneration and improve contractility of injured detrusor muscle and the urethral sphincter [48]. Therefore, injection of MSCs may represent a new treatment option for anal incontinence caused by sphincteric lesions [49]. The first steps toward animal experimentation recently have been taken, showing success through clinical, histological, and electromyographic evaluation when regenerating anal sphincter function that has been experimentally damaged [50, 51].
Indications and Contraindications of Treatment with Bulking Agents
Indications
Indications for this treatment include passive fecal incontinence to solid or liquid stool occurring once per week or more because of IAS dysfunction or related to simple or multiple IAS defects and with failure of conservative treatments (Fig. 33.3).
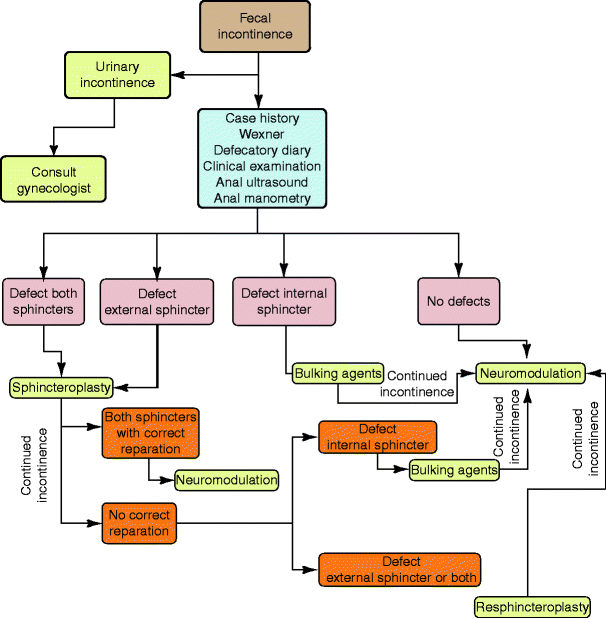

Fig. 33.3
Place of volume augmenters in the therapeutic algorithm for fecal incontinence
Contraindications
Rectal Disorders
Flatus incontinence only
External anal sphincter defect
Significant rectal or mucosal prolapse
Active anorectal sepsis
Current anorectal tumors
Current anal fissures
Rectal anastomosis at a level <10 cm from the anal margin
Active proctitis
Idiopathic anorectal bleeding, rectal varices, or vascular malformation
Anorectal stenosis
Significant chronic anorectal or pelvic pain
Hemorrhoidal disease grades III–IV
Anorectal malformation
Concurrent Disease/Concomitant Medications
Inflammatory bowel disease
Chronic diarrhea that is unresponsive to medical treatment
Medical history of human immunodeficiency virus infection or any serious immunocompromised state or administration of immunosuppressive therapy
Hemorrhagic diathesis or current anticoagulant therapy, such as warfarin, heparin, or substances similar to heparin
General
Pregnancy or within the first 6 months postpartum
Age younger than 18 years or older than 80 years
General Practical Aspects of Treatment with Bulking Agents
Injection Preparation
In general, regardless of the injection site (intersphincteric or submucosal), all bulking agents only need the preparation of the rectum with a simple phosphate enema at least 2 h before the procedure. Although some protocols suggest patients use laxative treatments at least 2 days before deployment to soften the stools, I do not consider it to be important for the success of the treatment. On the contrary, it can make the incontinence worse, causing perianal skin irritation, dermatitis, and risk of contamination. There is agreement on the necessity of administering antibiotic prophylaxis before the procedure, which can take the form of a first-generation cephalosporin (which covers the cutaneous flora) and metronizadole (anaerobic flora) [25, 26].
Injection Technique
The different agents can be injected either perianally, going through the muscle complex or just through the IAS, as well as submucosally (Fig. 33.4). The latter technique might have a higher risk of erosion and sepsis [52]. The implant can be placed in the intersphincteric plane or in the submucosal plane but always above the dentate line. Implants placed under the dentate line cause significant postoperative pain (Fig. 33.5). Implants can be injected in four or fewer quadrants or in only the IAS defect site to enhance the symmetry of the anal canal. The injection can be guided digitally (through direct visualization) or by endoanal ultrasound. It recently has been suggested that endosonographic guidance is associated with improved short-term continence [53], and there was previous concern that its use would disperse the implant [26]. The procedure usually is performed in an outpatient setting and can be conducted under local anesthesia with sedation; however, some patients do not need any sedation, so it could be performed in the doctor’s office as an ambulatory procedure (Fig. 33.6).
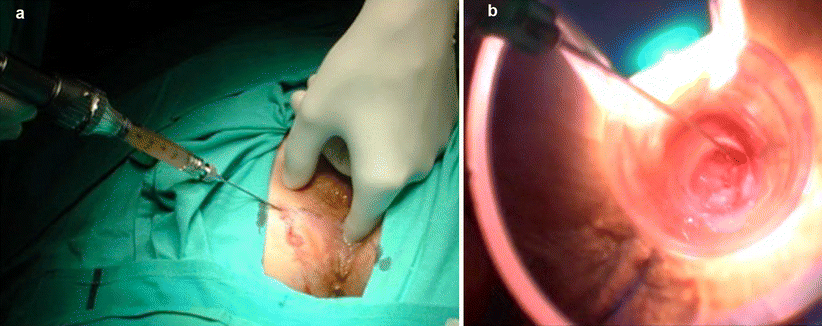
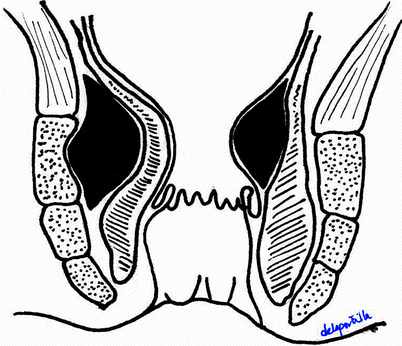
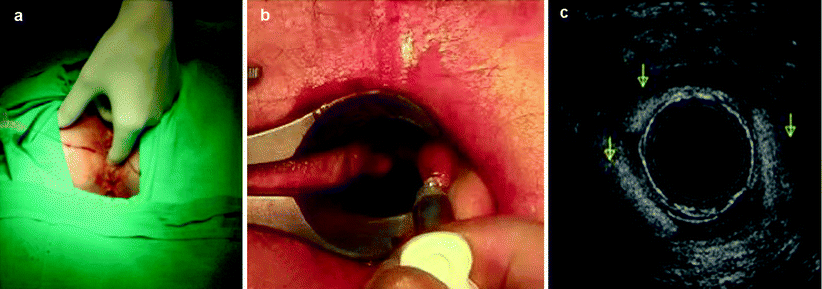

Fig. 33.4
Means of injection of bulking agents. (a) Perianal injection. (b) Submucosal injection

Fig. 33.5
Location of the implant, right-hemisphere submucosal location and the left-hemisphere intersphincteric location of the injected agent

Fig. 33.6
Different injection methods. (a) Digital guidance. (b) Through direct visualization. (c) Endoanal ultrasound guidance
The volume of the injection depends upon the agent employed and the injection method chosen (Table 33.1). To date, no study compares the number and location of the implants and the effectiveness of different treatment protocols [54].
Table 33.1
Means of administration, location of the implant, and volume of the main bulking agents currently on the market
Agent | Injection route | Location of implant | Total volume |
|---|---|---|---|
Teflon | Submucosal | Submucosal | 10 mL |
Autologous fat | Submucosal | Submucosal | 15–20 mL |
PTQ Implants | Perianal | Submucosal or intersphincteric | 7.5 mL |
Contigen | Submucosal | Submucosal | 2–5 mL |
Durasphere | Submucosal or perianal | Submucosal or intersphincteric | 8–10 mL |
Coaptite | Perianal | Submucosal | 4 mL |
Solesta | Submucosal | Submucosal | 4 mL |
Permacol | Perianal | Submucosal | 15 mL |
Bulkamid | Perianal | Submucosal | 9 mL |
Urosurge | Submucosal | Submucosal | Up to 5 balloons |
Outcome
A recently published systematic Cochrane review analysis designed to assess the effectiveness of volume augmentations showed a shortage of well-designed randomized trials. Only one of the four selected randomized trials had an adequate sample size, whereas the other trials assessed in the review showed inadequate sample sizes, a lack of controls, lack of blind assessments, or an incorrect notification about the patients’ follow-up [53].
There is a great variety of observational studies showing a statistically significant short-term improvement, although there are wide variations in the agents and techniques used. The global assessment of the published studies seems to show that most of the agents are relatively safe; although complications are common, they are relatively insignificant and transitory with most of the agents used. However, in view of the currently poor quality and availability of information, these conclusions can be only provisional.
Although many published studies comparing the different agents are anecdotal, some materials such as silicone seem to be more efficacious than others, at least in the short term [55]. Silicone (PTQ Implants) have been the most widely used material and could be defined as the gold standard treatment at this time. In this respect, however, only one controlled trial has shown superiority of PTQ Implants when compared with Durasphere in terms of efficacy, along with fewer complications [55]. The only placebo-controlled trial available that compared a saline solution treatment to treatment with PTQ Implants did not show any significant statistical difference between patient cohorts [56]. There is still a lack of accurate information regarding the volume, the precise location where the agent should be injected, and the means of implantation. A single study evaluated the long-term effectiveness in six patients [57], and at a 61-month follow-up, one patient had undergone a colostomy for fecal incontinence. The incontinence score of the remaining five patients was essentially unchanged, but there was a substantial improvement in physical and social functioning on the 36-item Short Form scores, and satisfaction scores were high. Subjectively, three patients were improved: one of these had undergone a further set of injections and one improved after a course of biofeedback. After the follow-up period, one of the five patients had a colostomy for an associated rectovaginal fistula. The study conclusion was that the effectiveness of the treatment with PTQ Implants for passive fecal incontinence varies over time and that most of the patients probably will need to be reinjected to maintain the improvement.
Despite that there is currently no clear evidence concerning the effectiveness of the volume augmenter agents, there is no doubt they can be useful in some patients with passive fecal incontinence as a first-line, minimally invasive alternative after failure of a medical or conservative treatment. Patients should be informed that the majority of cases recording improvement will need further treatments (Table 33.2).
Table 33.2
Summary of the studies of bulking agents to date
Author | Agent | n | Improved (%) | Follow-up (mo) | |
|---|---|---|---|---|---|
Shafik [18] | Teflon/Polytef | Submucosal | 11 | 50 | 22 |
Shafik [23] | Autologous fat | Submucosal | 14 | 100 | 6 |
Bernardi [58] | Autologous fat | Circumferential | 1 | 10 | 12 |
Feretis [46] | Microballons |









