Author (year)
No. of patients
Recurrence (%)
Ripstein procedure
Ripstein (1972) [13]
289
0
Biehl et al. (1978) [14]
22
10
Gordon and Hoexter (1978) [15]
1111
2.3
Eisenstat et al. (1979) [16]
30
0
Failes et al. (1979) [17]
53
5.7
Romero-Torres (1979) [18]
24
0
Morgan (1980) [19]
64
1.6
Roberts et al. (1988) [20]
135
9.6
Leenen and Kuijpers (1989) [21]
64
0
Tjandra at al. (1993) [1]
134
8
Winde et al. (1993) [22]
35
0
Schultz et al. (2000) [23]
105
2
Ivalon sponge procedure
Morgan et al. (1972) [24]
150
3.2
Penfold and Hawley (1972) [25]
101
3
Stewart (1972) [26]
41
7.3
Boutsis and Ellis (1974) [27]
26
11.5
Anderson et al. (1984) [28]
42
2.4
Atkinson and Taylor (1984) [29]
40
10
Boulous et al. (1984) [30]
32
15.6
Kuijpers and de Morree (1988) [31]
30
0
Arndt and Pircher (1988) [32]
62
6.4
Yoshioka et al. (1989) [33]
165
1.5
Sayfan et al. (1990) [34]
16
0
Luukkonen et al. (1992) [35]
15
0
Novell at al. (1994) [36]
31
3.2
Abdominal rectopexy
Loygue et al. (1971) [37]
140
3.6
Blatchford et al. (1989) [38]
42
2
Solomon and Eyers (1996) [39]
45
0
Boccasanta et al. (1999) [40]
23
13
Solomon et al. (2002) [41]
39
2.5
Byrne et al. (2008) [42]
321
4
Abdominal rectopexy and sigmoid resection
Watts et al. (1985) [43]
102
1.9
Husa et al. (1988) [2]
48
9
Sayfan et al. (1990) [34]
13
0
McKee et al. (1990) [44]
9
0
Luukkonen et al. (1992) [35]
15
0
Huber et al. (1995) [45]
39
0
Xynos et al. (1999) [46]
18
0
Perineal rectosigmoidectomy
Altemeier et al. (1971) [47]
106
3
Gopal et al. (1984) [48]
18
6
Finlay and Aitchison (1991) [49]
17
6
Williams et al. (1992) [50]
114
11
Johansen et al. (1993) [51]
20
0
Author (year)
No. of patients
Recurrence (%)
Agachan et al. (1997) [52]
32
13
Kim (1999) [53]
183
16
Azimuddin et al. (2001) [54]
36
16
Kimmins (2001) [55]
63
16
Schutz (2001) [56]
31
0
Zbar et al. (2002) [57]
80
4
Delorme procedure
Uhlig and Sullivan (1979) [58]
44
7
Monson et al. (1986) [59]
27
7
Graf et al. (1992) [60]
14
21
Senapati et al. (1994) [61]
32
13
Oliver et al. (1994) [62]
41
22
Tobin and Scott (1994) [63]
43
26
Liberman et al. (2000) [64]
34
0
Watts et al. (2000) [65]
101
27
Watkins et al. (2003) [66]
52
10
Thiersch procedure
Jackaman et al (1980) [67]
52
33
Labow et al. (1980) [68]
9
0
Hunt et al. (1985) [69]
41
44
Poole et al. (1985) [70]
15
33
Vongsangnak et al. (1985) [71]
25
39
Earnshaw and Hopkinson (1987) [72]
21
33
Khanduja et al. (1988) [73]
16
0
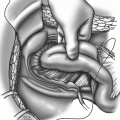
More commonly performed abdominal repairs of rectal prolapse include rectopexy, with or with resection of the redundant sigmoid colon. Rectopexy with resection of bowel is indicated in patients who have a history of chronic constipation. Husa et al. [2] reported results from 48 patients who underwent rectopexy and sigmoid resection. At a mean follow-up of 4.3 years (range, 1–10 years), recurrence occurred in 9 % of patients. Although there is a theoretical advantage of greater fixation after rectopexy with resection due to fibrosis between the colonic anastomosis and the sacrum, the recurrence rates after rectopexy with resection do not differ significantly from rectopexy alone. Results after a laparoscopic approach of either procedure have found similar success rates [3–7].
The two most commonly performed perineal procedures are the Delorme procedure and the perineal rectosigmoidectomy. The Thiersch procedure, which involves encircling of the anal canal with a synthetic mesh, does not eradicate prolapse but merely prevents its further descent by narrowing the anal canal and providing mechanical support. This procedure was found to have a high recurrence rate (up to 44 %) and therefore largely has been abandoned.
The Delorme procedure involves the excision of the mucosa and submucosa of the prolapsed segment with a plication of the muscularis propria. Recurrence rates after this procedure range from 0 to 27 %. Perineal rectosigmoidectomy (the Altemeier procedure) involves a full-thickness excision of the rectum and a portion of the sigmoid colon. Recurrent prolapse after perineal rectosigmoidectomy occurs less frequently than after the Delorme procedure but may still occur in up to 16 % of patients.
Although there is a breadth of experience described in the literature regarding the initial management of full-thickness rectal prolapse, there are only a few studies that specifically report on the treatment of patients with recurrent disease. Hool et al. [8] reported the experience at the Cleveland Clinic Foundation regarding the treatment of 24 patients with recurrent rectal prolapse. Nine patients originally had undergone a perineal repair whereas 15 had an abdominal repair (ten of these were Ripstein repairs). Treatment of the recurrences included 25 abdominal repairs and four perineal operations. Although the authors did not specifically address re-recurrence, one patient required three abdominal procedures. The cause of the recurrence was able to be identified in only 12 patients and was mainly related to failure of the mesh after Ripstein repairs. The mean interval to the initial recurrence was 2 years; however, 34 % of patients presented with recurrence within 7 months of the initial procedure.
Fengler et al. [9] presented results of the management of 14 patients with recurrent rectal prolapse. The majority of the patients in this series had undergone a perineal repair as the initial operation. These included perineal proctectomy and levatorplasty in ten patients, anal encirclement in two patients, a Delorme procedure in one patient, and an anterior resection in one patient. The mean time to recurrence was 14 months. In patients who had undergone a perineal proctectomy, five underwent a repeat perineal proctectomy, four underwent rectopexy (one with combined resection), and one underwent anal encirclement. The patient with recurrence after anterior resection underwent a Delorme procedure and the patient who had initially undergone a Delorme repair underwent a perineal proctectomy. The mean length of follow-up was 50 months and none of the patients in this series had a re-recurrence.
Pikarsky et al. [10] compared the outcomes of 27 patients with recurrent prolapse with 27 matched patients with primary rectal prolapse. The initial operation in the recurrent prolapse group was rectopexy in seven patients, a Delorme procedure in seven patients, perineal rectosigmoidectomy in seven patients, anal encirclement in four patients, and resection rectopexy in two patients. Procedures performed for recurrent prolapse included perineal rectosigmoidectomy in 14 patients, resection rectopexy in 8 patients, rectopexy in 2 patients, anal encirclement in 2 patients, and a Delorme procedure in 1 patient. There was no significant difference in the recurrence rates (14.8 % in the recurrent group vs. 11.1 % in the primary group). The authors concluded that the outcome of surgery for rectal prolapse is similar in cases of recurrent or primary prolapse and that the options for surgery are equivalently valid in both scenarios.
The largest series available in the literature was presented by Steele et al. [11] from the University of Minnesota. The authors identified 78 patients who underwent repair of recurrent rectal prolapse. The majority (61 of 78) of the patients with recurrence had undergone a perineal repair as the initial operation. The mean interval to the first recurrence in this series was 33 months, with 29 % of the recurrences presenting within the first 7 months. Fifty-one patients underwent a perineal repair for recurrent rectal prolapse, whereas 27 had an abdominal approach. Re-recurrence occurred in 23 patients (29 %) at a mean follow-up of 9 months. Re-recurrence was significantly higher after reoperative surgery involving a perineal procedure (19 of 51 patients; 37.3 %) when compared with an abdominal repair (4 of 27 patients; 14.8 %). Six of the 19 patients with re-recurrence after a perineal repair underwent a repeat perineal procedure, two of whom (33 %) developed a third recurrence. Eleven patients with re-recurrence after a perineal repair subsequently underwent an abdominal procedure, and a third recurrence occurred in 9.1 % of these patients. When the authors combined the recurrence rates after the first, second, and third recurrences, the abdominal approach had a significantly lower recurrence rate (13 %) when compared with the perineal approach (39 %).
Repair of recurrent rectal prolapse also has been described using a laparoscopic approach. Tsugawa et al. [12] reported performing laparoscopic rectopexy on two patients with recurrent prolapse. Both patients had previously undergone a Gant-Miwa operation, a procedure commonly performed in Japan that involves a plication of the prolapsed rectal mucosa along with anal encirclement. The authors used a suture rectopexy technique and neither patient developed re-recurrence during a 2-year follow-up period.
It is important to reevaluate the patient with recurrent full-thickness rectal prolapse for constipation or incontinence symptoms as well as other pelvic floor abnormalities. Anorectal physiology studies such as defecography and anal manometry may be useful, as well as flexible endoscopy to exclude concomitant pathology. The age and general health of the patient is also important in the selection of the optimal treatment. A careful review of the operative report of the primary repair is also necessary. There are no hard and fast recommendations concerning the evaluation of patients presenting with either primary or recurrent rectal prolapse. In children, the management is directed more toward conservative therapies and the treatment of underlying constipation, with either of ultrasound or magnetic resonance proctography used in defining rare cases with megarectum [74]. In those in whom a redo perineal rectosigmoidectomy is contemplated, it is likely (although unproven) that the preoperative use of anorectal manometry may be valuable; the failure of elicitation of a rectoanal inhibitory reflex, low preoperative resting anal pressures, or both probably preclude the performance of a perineal rectosigmoidectomy as part of a repeat operation [57,75,76]. The complex physiology after rectal prolapse repair is poorly understood; there will be changes in neorectal compliance, improvements in mucosal rectal sensitivity, sphincter damage secondary to the prolapse itself, alterations in internal anal sphincter relaxation, improved rectoanal propulsive coordination, and an abrogation of massive rectal motor waves often seen preoperatively [57,77]. There is little evidence that there are predictive factors that define cases where prolapse repair still leaves the patient incontinent [78]. Here, surgical “cure” of the prolapse alone is associated with improvement in continence in 80 % of patients who present with preoperative incontinence [79]. Although it would seem logical to assess sphincter morphology with preoperative endosonography, there is no objective trial evidence that the performance of an attendant levatorplasty improves outcome of the primary or secondary prolapse repair [80].
Determining the best option for repair of recurrent prolapse depends largely on the nature and conduct of the initial operative procedure. Residual blood supply of the remaining colon and rectum may be significantly compromised in patients who undergo repeat resections. Patients may suffer from ischemia of the segment of intestine between the two anastomoses—a situation which should be avoided. This problem can occur either at sigmoid resection after an initial perineal rectosigmoidectomy or at perineal rectosigmoidectomy after an initial sigmoid resection, unless the surgeon is confident that the anastomosis (particularly in the latter scenario) will be part of the repeat resection. Repeat perineal rectosigmoidectomy can be performed safely, again ensuring that the previous anastomosis in the rectum is resected [11]. A Delorme procedure is another option for patients with recurrent prolapse after a prior resectional procedure. Rectopexy without resection may be difficult in patients who have previously undergone a perineal rectosigmoidectomy because there may be insufficient lateral attachments to secure to the sacrum. Furthermore, a laparoscopic approach to failed abdominal rectopexy will be particularly challenging, even in the hands of a surgeon experienced in laparoscopic sacrorectopexy [41]. After abdominal mesh rectopexy (which is hardly being used currently), nearly half of the recurrences were due to technical aspects related to mesh deployment, where it had detached from the rectum or the sacrum or where it was applied too loosely or too low on the rectal wall [8]. The management of children with primary and recurrent rectal prolapse is beyond the scope of this chapter, but a range of procedures that are not utilized in adults are employed, including transsacral rectopexy (the Ekehorn procedure) [81,82] and posterior sagittal anorectoplasty [83]. In this group of patients, rectal prolapse is most commonly seen in children younger than 4 years of age, with the highest incidence during the first year of life [84]. Many treatments are conservative; most recurrences noted occur in children outside this typical age range and surgical therapy is more often required, the prolapse is often more severe, and consideration should be given to an “adult surgical approach.” A treatment algorithm for full-thickness rectal prolapse is summarized in Fig.51.1.
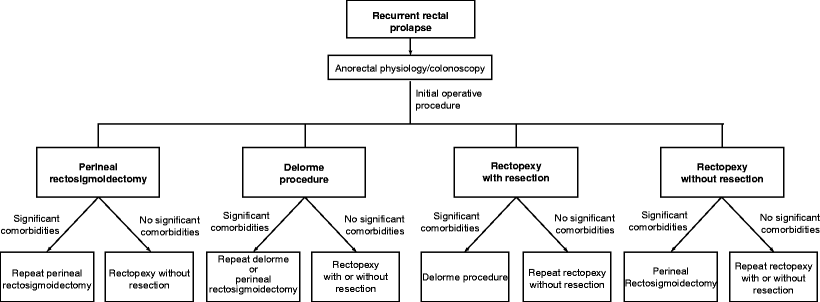

Fig. 51.1
A treatment algorithm for full-thickness rectal prolapse
Recurrence after operative repair of full-thickness rectal prolapse occurs quite frequently, particularly after a perineal procedure. Re-recurrence rates also remain high, which may signal that the underlying pathology is not being adequately treated with currently available procedures. Despite this, reoperation does have success in patients with recurrent rectal prolapse, particularly with abdominal repairs [85]. The role of preoperative imaging and physiological assessment is unknown, although it would seem intuitive to document the presence of a significant sphincter injury that may potentially require treatment at a later date in those in whom incontinence persists after surgery. The introduction of laparoscopic procedures and some novel technologies (including nerve-sparing ventral rectopexy in constipated patients or transvaginal sacrospinous rectopexy in incontinent cases) [86–89] has made the decision for standard transanal perineal procedures more complicated; minimally invasive access may permit frailer patients to undergo abdominal procedures more readily [90]. In this regard, prospective controlled trials are required, although these will be difficult to randomize given the variable patient comorbidity and age [91].
Conclusion
It is important that each patient be assessed individually because the selection of the best specific procedure for a given patient remains highly individualized. Coloproctologists must await the longer-term outcome recurrence data in longitudinal, single-arm studies using novel techniques before deciding in selected circumstances where there is a complex presentation with chronic constipation, fecal incontinence, and/or multicompartment pelvic organ prolapse about their individual role either in primary or recurrent rectal prolapse. In these patients only sustainable success (in terms of recurrent disease) will be associated with improvements in quality of life [92]. In such cases, application of an algorithmic approach will be difficult and there will need to be flexibility in the preoperative use of extended defecographic, transperineal ultrasonographic, and magnetic resonance proctographic techniques to determine the presence of other pelvic visceral and soft-tissue abnormalities. It may well be that in some of these patients, a multidisciplinary operative approach will be required [93–95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree