(1)
Division of Dermatology, Department of Medicine, David Geffen School of Medicine at UCLA, 52-121 Center for Health Science, Los Angeles, CA 90095, USA
(2)
Division of Dermatology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Abstract
The skin is traditionally viewed as a physical barrier to environmental insults. Its role in immunity goes beyond its role as simply a barrier, as it plays a dynamic role in regulating immune responses and controlling microbial populations, notably through the use of antimicrobial peptides (AMPs). In 1987, Zasloff and colleagues were one of the first to describe the existence of potent AMPs on the skin of vertebrates. Since then, numerous AMPs have been identified and characterized in human skin. AMPs are a heterogeneous group of small proteins with a wide spectrum of antimicrobial activity against bacteria, fungi, and viruses. These peptides are almost all positively charged and amphipathic, allowing for the peptides to be soluble in an aqueous environment while still retaining an ability to bind bacterial membranes and walls. Once bound to the target membrane, the peptides kill through various mechanisms that involve both the physical perforation of pathogens and triggering of the host immune response. This chapter will focus on known AMPs present in human skin, with special focus given to defensins, cathelicidins, granulysins, S100 proteins, and ribonucleases; and their involvements in the etiology of dermatologic diseases.
Keywords
Antimicrobial peptide (AMP)DefensinCathelicidinLL-37GranulysinS100Ribonuclease (RNase)PsoriasinDermcidinSecretory leukocyte protease inhibitor (SLPI)PsoriasisAtopic DermatitisAcneRosaceaMycobacteriaWound healingIntroduction
The skin is primarily thought of as a physical barrier to environmental insults and microbes. Yet its role extends far beyond simply a physical barrier, as the skin is also a dynamic immune organ that responds with robust defense mechanisms to microbial invaders, notably through the use of antimicrobial peptides (AMPs). AMPs in the skin are of considerable interest not only for their properties in controlling microbial populations but also for their effects on influencing inflammatory and immune responses. In 1987, Zasloff led one of the first groups to describe the existence of a potent antibacterial peptide present in the skin of the African frog Xenopus laevis [1]. Since then, numerous AMPs have been described in human skin and their roles in host defense have been extensively characterized.
AMPs are a heterogeneous group of small molecular weight proteins with a wide spectrum of antimicrobial activity against bacteria, fungi, and viruses. These peptides are almost all positively charged and amphipathic, having both hydrophobic and hydrophilic surfaces, allowing for solubility in aqueous environments while still retaining the ability to bind bacterial membranes through their hydrophobic surfaces. Once bound to the target membrane, the peptides can kill the organism through various mechanisms. Furthermore, there is a growing body of evidence supporting the ability of AMPs to alter the host immune response (Table 6.1).
Table 6.1
Antimicrobial and immunomodulatory properties
Antimicrobial activity | Regulation | |||
|---|---|---|---|---|
Gram + | Gram − | Fungi | ||
α-defensin | + | + | + | Constitutive |
HBD-1 | ± | + | − | Constitutive |
HBD-2 | ± | + | + | Inducible via IL-1/NF-kB dependent mechanism Toll-like receptors |
HBD-3 | + | + | + | Inducible via TGF-α, and IGF-1 |
HBD-4 | + | + | + | Via NF-kB independent pathways |
Cathelicidin | + | + | + | Constitutive and inducible Vitamin-D Toll-like receptors |
Granulysin | + | + | + | Toll-like receptors Activator protein-1 dependent pathway |
Psoriasin | ± | + | − | Calcium All-trans retinoic acid Inflammatory stress UV light EGFR ligands IL-1 |
Dermcidin | + | + | + | Constitutive |
RNAse 7 | + | + | + | Constitutive and inducible Inducible via UVB, IL-1β, IFN-γ, TNF-α Bacterial challenge (S. aureus, P. aeruginosa) |
This chapter will focus on the main AMPs present in human skin and the relevant dermatologic conditions in which deficiency or overexpression of AMPs is thought to play an important etiologic role. (A summary of described AMPs present in the skin can be found in Table 6.2.)
Table 6.2
Summary of major antimicrobial peptides present in skin
Cell source | Other properties | |
|---|---|---|
α-defensins | Neutrophils | Increase (TNF)-α and interleukin (IL)-1 in S. aureus activated monocytes Inactivation of adenovirus and polyomavirus |
β-defensins | Keratinocytes | Chemotaxis of T cells and Dendritic cells |
Cathelicidin (LL-37) | Keratinocytes Ductal epithelium Eccrine glands Mast cells Nail bed | Angiogenesis Wound healing Chemotaxis of neutrophils, monocytes, T cells, and mast cells Induction of IL-1β and IL-6 secretion in keratinocytes Inhibition of IAP-2 via COX-2 Anti-biofilm effects |
Granulysin | Cytotoxic T lymphocytes NK cells | Chemotaxis of T cells, monocytes, NK cells, and dendritic cells Cytotoxic to tumor cells Anti-inflammatory Graft Rejection Induction of MCP-1, MCP-3, IL-1, IL-6, IL-10, and IFN-α in monocytes Mediator of keratinocyte cell death in SJS and TEN |
Psoriasin | Keratinocytes Follicular epithelium Sebocytes | Chemotaxis of CD4+ lymphocytes and neutrophils Calcium-dependent oleic acid transport and metabolism |
Dermcidin | Eccrine glands | Limits bacterial colonization |
RNase 7 | Keratinocytes | Antimicrobial properties against Gram + and Gram − organisms Antifungal properties |
Defensins
Defensins are a family of AMPs with a characteristic β-sheet fold and six disulfide bonds between highly conserved cysteine residues. There are two main defensin subfamilies, alpha- and beta-defensins, that differ mainly in the pairing of cysteine residues. Members of both subfamilies consist of a triple-stranded β-sheet with a prototypic ‘defensin’ fold (Fig. 6.1). There is a third defensin family, theta-defensins, that is not expressed in humans but is found in several Old World primates. Theta-defensins are thought to inhibit the fusion of HIV-1 to host cells by inhibiting bundle formation needed for fusion [2, 3].
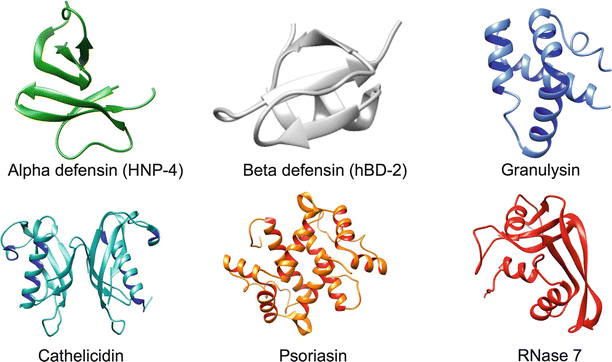

Fig. 6.1
Representative crystal structures of major antimicrobial peptides. Shown here are representative crystal structures of alpha defensin, beta defensin, granulysin, cathelicidin, psoriasin, and RNase 7. Alpha defensin, beta defensin, granulysin, and RNase 7 are shown as monomers. Cathelicidin and psoriasin are shown as dimers
Defensins are widely distributed in cells and tissues involved in host defense and are found in highest concentrations within phagocyte granules. Alpha-defensins, which have disulfide bridges between cysteines 1–6, 2–4, and 3–5 [4], are found predominantly in neutrophils [5], and have aptly been named human neutrophil peptides (HNPs). In humans, alpha-defensins are stored in azurophilic granules of neutrophils as fully processed mature peptides. Two alpha-defensins, human defensins (HD)-5 [6] and 6 [7], are expressed in Paneth cells of the small intestine and the epithelium of the female urogenital tract [8].
Alpha-defensins show a wide spectrum of antimicrobial activity against bacteria and fungi. They have also been shown to inactivate certain viruses, including adenovirus [9] and polyomavirus [10], and have been implicated as one of the molecules that may be important in the antiviral activity seen in CD8+ T cells of HIV-non-progressors [11]. Immunologically, alpha-defensins contribute to the host inflammatory response, for example, by increasing the expression of tumor necrosis factor (TNF)-α and interleukin (IL)-1 in S. aureus-activated monocytes [12]. High concentrations of alpha-defensins are toxic to mammalian cells and may be important in tissue injury and necrosis during inflammation. It has been suggested that the upregulation of alpha-defensins 1–3 from T cells may be involved in the etiopathology of Stevens-Johnsons Syndrome and toxic epidermal necrolysis [13].
Human beta-defensins (hBDs) are also characterized by six cysteine motifs, but are distinct from alpha-defensins through their cysteine cross-bridging. The disulfide bonds of hBDs are instead between cysteines 1–5, 2–4, and 3–6 [14]. hBDs-1, -2, -3, and -4 have been identified in many cell types, including epithelial cells, but their expression, localization, and antimicrobial specificities vary. hBD-1 is expressed predominantly in the urinary tract but is also expressed constitutively in the skin. hBD-2 is largely absent in healthy skin but is induced by bacteria through an IL-1/NF-kB dependent mechanism [15, 16]. The antibacterial spectrum of hBD-2 is broad, encompassing both Gram-negative and Gram-positive organisms. Studies found that hBD-2 is bactericidal against Pseudomonas aeruginosa, Fendegoldia magna, and Streptococcus pyogenes, while only bacteriostatic against Staphylococcus aureus [17, 18]. A synergistic effect of IL-1α and epidermal growth factor receptor (EGFR) ligands has been proposed in the induction of hBD-2 [19]. hBD-3 is also a broad spectrum antibiotic for a wide range of bacteria and fungi [20]. As with hBD-2, hBD-3 induction has also been proposed to be dependent on the transactivation of EFGR, yet with its own set of growth factors, including TGF-α and IGF-1 [21]. In addition to its bactericidal effects, hBD-3 has been shown to induce phenotypic maturation of Langerhans cell-like dendritic cells [22]. Lastly, hBD-4 induction is suggested to involve NF-kB independent pathways that have yet to be fully characterized [23].
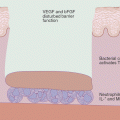
Both alpha-and beta defensins share a common antimicrobial mechanism. The leading hypothesis proposes that the permeabilization of target membranes is the critical step in defensin-mediated cytotoxicity. In experimental models using Escherichia coli, membrane permeabilization resulted in the subsequent inhibition of bacterial metabolism, including RNA, DNA, and protein synthesis (Fig. 6.2) [24]. One study proposed the formation of a stable 25 Å pore, comprised of a hexamer of defensin dimers [25], allowing for small intracellular molecules to leak out of the organism resulting in a decrease in viability. This mechanism, however, only partially explains the antimicrobial activity, as evidence exists for both a transient pore formation and also intracellular sites of action, which may also be important in cell death [26]. In addition, some defensins have been found to bind to membrane glycoproteins with high affinity, giving rise to a possible explanation for antiviral activity [27]. Other proposed mechanisms of action of defensins include modifying cell migration and maturation, inducing cytokines, and triggering histamine and prostaglandin D2 release from mast cells.
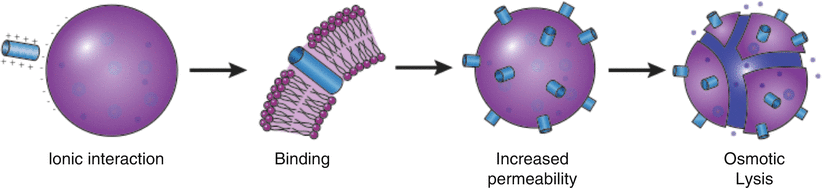

Fig. 6.2
Proposed mechanism of action of antimicrobial peptides. Cationic AMPs (e.g., granulysin) associate with the negative bacterial membranes, resulting in binding and subsequent increased permeability of the phospholipid bilayer. As a result of the increased permeability, irreversible osmotic damage results in cell death
Cathelicidins
Cathelicidins are a family of AMPs that contain a conserved cathelin domain, characterized by an N-terminal signal peptide, a prosequence, and a C-terminal cationic peptide [28]. Cathelicidins are expressed by cells in direct contact with the external environment. The propeptide hCAP18 (human cationic AMP, 18 kD) is first synthesized and stored in granules and lamellar bodies of keratinocytes. When hCAP18 is released into the extracellular environment, its antimicrobial C-terminus is cleaved by proteinase 3 in neutrophils and kallikrein in keratinocytes to produce the α-helical LL-37 (“LL” for two leucine residues, 37 for the number of residues present in the peptide) (Fig. 6.1) [29]. The human cathelicidin family is limited to just the one gene with a protein product hCAP18. It was initially identified in keratinocytes at the site of wound healing [30]. It was later found to be constitutively expressed in other locations and conditions, such as in nail beds, eccrine glands [31], and neonatal skin (Fig. 6.3) [32].
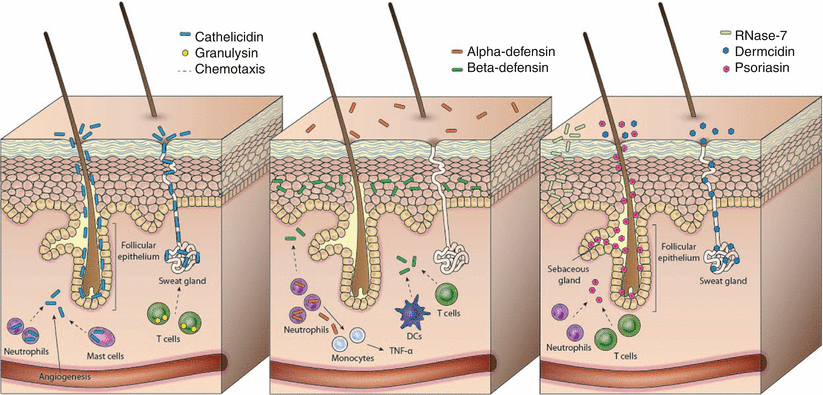

Fig. 6.3
Antimicrobial peptide expression in the skin. The expression of some AMPs by both resident skin cells and infiltrating immune cells is demonstrated in this schematic diagram
LL-37 has a broad antimicrobial spectrum against bacteria, fungi, and viruses. It is bactericidal against both Gram-positive and Gram-negative organisms including Listeria monocytogenes, S. aureus, S. epidermidis, Salmonella typhimurium, E. coli, and vancomycin-resistant Enterococci [33]. Unlike murine and reptilian cathelicidins, LL-37 is the only known cathelicidin with anti-biofilm properties against the opportunistic human pathogens P. aeruginosa and Francisella novicida [34]. Mice with a mutation in CRAMP (the murine homologue of LL-37) are more susceptible to Streptococcal infections when compared to wild-type controls [35]. In humans, LL-37 is further processed into smaller peptides, such as RK-31 and KS-30, which have increasing antimicrobial activity against Staphylococcal species [36]. LL-37 is also active against fungal species, most notably Candida albicans [37], by disturbing membrane morphology to allow for a swift influx of molecules with masses up to 40 kDa [38]. Furthermore, LL-37 has a broad antiviral spectrum and has been shown to be viricidal against the vaccinia virus [39], influenza virus [40], herpes simplex 1 virus [41], adenovirus 19 [41], varicella zoster virus [42], and HIV-1 [43].
The expression of LL-37 within human skin is both constitutive and inducible. In eccrine glands and ductal cells, LL-37 is diffusely expressed in the cytoplasm of secretory glands and also the ductal epithelium [31]. Serine proteases present within sweat further cleave LL-37 into smaller peptides giving rise to enhanced antimicrobial activity against Staphylococcal and Candidal species [36]. LL-37 expression is induced in patients with cutaneous lupus erythematosus [44], and is upregulated during wound healing. Studies using a cultured keratinocyte model suggest the importance of IGF-1 in the latter scenario [45].
Vitamin D is frequently mentioned in discussions about LL-37 in the skin as an important regulator. The gene encoding LL-37 contains a vitamin D response element (VDRE) present in its promoter, which explains the dependence of LL-37 expression on vitamin D and its precursors [46]. Liu et al. [47] found that Toll-like receptor (TLR)-2-dependent microbicidal activity was dependent on the vitamin-D induced expression of LL-37 [47]. This identified a novel mechanism for TLR-induced antimicrobial activity and may represent one of the main defenses of infection for both cutaneous and systemic tuberculosis. The relationship between vitamin D and LL-37, however, remains unclear, as calicipotriol, a synthetic form of vitamin D, downregulates LL-37 in keratinocytes prestimulated with UVB, LPS, and TNF-α [48]. This dichotomous relationship poses the hypothesis that vitamin D-induction of LL-37 depends on whether or not the condition in question inflammatory or non-inflammatory.
As with other AMPs, LL-37 also has a role in modulating the host immune response. LL-37 is chemoattractant for neutrophils, monocytes, and T cells by binding formyl-peptide-receptor-like (FPRL)-1. In addition, it recruits and stimulates subsequent production of LL-37 via mast cells, creating a positive feedback loop [49]. In keratinocytes, LL-37 not only has a role in inducing IL-1β and IL-6 secretion [36], but also inhibits the expression of inhibitor of apoptosis-2 (IAP-2) via cyclooxygenase-2 (COX-2), inhibiting apoptosis [50].
Granulysin
Granulysin is an AMP derived from cytotoxic T lymphocytes and NK cells and belongs to a member of the larger saposin-like protein family. Unlike defensins and cathelicidins that are expressed in epithelial cells, granulysin is found exclusively within the granules of cytotoxic T and NK cells recruited to sights of inflammation. Granulysin co-localizes to the cytotoxic vacuole with perforin and granzymes and acts synergistically to kill intracellular bacteria [51, 52]. Perforin forms pores in the cellular membrane allowing granulysin to access the intracellular compartment in which the pathogen resides, providing a direct mechanism of intracellular pathogen targeting (Fig. 6.3).
Granulysin is only found in humans and is synthesized as a 15 kD protein that is then cleaved to release the final 9 kD peptide [53]. The resultant 9 kD peptide is composed of 5 α-helices joined by short loops (Fig. 6.1) [54]. Granulysin is effective against a wide range of Gram-positive and Gram-negative bacteria, parasites, and fungi. Notably, it has been shown to be antimicrobial against Mycobacterium tuberculosis [52], Cryptococcus neoformans [55], Plasmodium falciparum [56], and Leishmania [57]. Granulysin also induces apoptosis of varicella-infected cells [58]. Although the structure of granulysin is significantly different from that of defensins or cathelicidin, it retains the conserved amphipathic nature. The hydrophobic surface is capable of associating closely with the target membrane, and the positively charged surface allows for association with the negatively charged bacterial membrane. In addition, studies on granulysin have shown the importance of the helix-loop-helix domain in the secondary structure. The ability of granulysin to kill S. typhimurium and E. coli has been localized to helix 2 and 3 of granulysin [57]. The amino acid residues contained within this structural component of granulysin is critical to the antimicrobial activity [59].
Similar to other AMPs, granulysin contains numerous immunomodulatory properties. Several studies show that granulysin is a chemoattractant for monocytes, NK cells, monocyte-derived dendritic cells, and a subset of T cells, including CD45Ro+ memory CD4 and CD8 T cells. Granulysin also induces the expression of multiple inflammatory cytokines in monocytes including MCP-1, MCP-3, IL-1, IL-6, IL-10, and IFN-α [60].
S100 Proteins
S100 proteins belong to a multigene family of proteins with numerous functions including keratinocyte differentiation, epithelial defense, and wound healing. They were first described by Moore et al. in 1965 as nerve-specific molecules from cattle brains “soluble in 100 % ammonium sulfate,” thus named S100 [61]. In general, S100 proteins are low molecular weight proteins (9–13 kD) of four conserved α-helical segments, two calcium binding regions, a central hinge, and an amino and carboxy terminal variable domain (Fig. 6.1). Interest in S100 proteins in the skin initially arose in part because many of the genes encoding for this family are located in the epidermal differentiation complex (EDC) [62], and have been implicated in epidermal defense.
In 1990, Celis et al. discovered an intense expression of low molecular weight proteins in the keratinocytes of psoriasis patients [63]. Psoriasin (S100A7) is an S100 AMP widely expressed in the human epidermis. While named after psoriasis, psoriasin is constitutively expressed on the surface of the skin and is also present in other skin diseases characterized by inflammation including lichen sclerosus and atopic dermatitis. Immunohistochemical staining has shown that psoriasin is expressed focally in keratinocytes with high expression in the stratum granulosum and stratum spinosum. As a peptide with antimicrobial properties, it is present in higher levels in areas with high bacterial colonization such as the face, axilla, and palms, and in areas with a high density of sebaceous glands and hair follicles (Fig. 6.3) [16, 64]. In addition to keratinocytes, studies have also shown staining in sebocytes, indicating secretion of psoriasin into sebum [64]. Psoriasin is thought to be preferentially active against E. coli, but also has some bactericidal activity against P. aeruginosa and S. aureus – albeit when psoriasin is at higher concentrations. Psoriasin is also found in utero and is thought to protect embryos from infection [65].
The bactericidal properties of psoriasin are attributed to the sequestration of Zn2+ [66]. Mutation experiments of calcium-binding and zinc-binding motifs on psoriasin confirm the importance of zinc deprivation in its killing mechanism [67]. Unlike other AMPs, the antimicrobial activity of psoriasin does not depend primarily on forming perforations in bacterial membranes. At pH values less than 6, psoriasin has been shown to permeabilize the bacterial membrane of Gram-negative E. coli and Gram-positive Bacillus megaterium; however, at neutral pH, the bacteria were killed without disrupting membrane structure in only E. coli and not B. megaterium [68].
In addition to its antimicrobial activity, psoriasin has numerous other features. Immunologically, it functions as a chemoattractant for CD4+ T cells and neutrophils [69]. Various studies have shown that epidermal production of psoriasin is inducible by many factors such as a through a synergistic induction by EGFR ligands and IL-1 [19], the binding of E. coli flagellin to TLR5 [70], and the binding of Th17 cytokines that can be suppressed by vitamin D [71]. Furthermore, UV and all-trans-retinoic acid have been shown to be exogenous regulators of psoriasin expression. Psoriasin has also been suggested to have metabolic effects as it is thought to interact with epidermal fatty acid binding protein to modulate calcium-dependent oleic acid transport and metabolism [72].
Other than psoriasin, there are 20 other known S100 proteins in the skin, 11 of which are expressed in keratinocytes including psoriasin (S100A2, S100A3, S100A4, S100A6, S100A8, S100A9, S100A10, S100A12, and S100A15), 1 in Langerhans cells and melanocytes (S100B), and 1 in Meissner’s corpuscles (S100P). S100A2 is localized primarily in the basal layer of the epidermis and in hair follicles and has been shown to be an early marker of oxidative stress [73]. S100A8 and S100A9 form both homo- and heterodimers and are frequently co-expressed. They have low expression in the epidermis and are sometimes found in the granular layer. The heterodimer of S100A8 and S100A9, also known as calprotectin, has some antifungal activity against C. albicans [74, 75]. Increased expression of these two S100 proteins is seen in wound healing and psoriasis. S100A10 is found both in the basal and spinous layers of the epidermis [76], and forms a homodimer that binds to a pair of annexin II to form calpactin I heterotetramer [77]. It is thought to be involved in the regulation of cell membrane formation during keratinocyte differentiation. The C-terminal peptide fragment of S100A12 (calcitermin) is capable of killing Gram-negative organisms showing in vitro activity against E. coli, L. monocytogenes, and C. albicans under acidic conditions [78]. Although initially described in human airway secretions, S100A12 is also expressed in both basal and suprabasal keratinocytes and is seen in psoriatic skin [79].
Ribonuclease
The RNase A superfamily contains 6–8 conserved cysteine residues forming disulfide bridges, conserved histidines, and a lysine at the center of ribonuclease activity [80]. The human RNase A superfamily currently has 13 known genes, of which 8 genes (RNases 1–8) have been shown to be catalytically active against RNA substrates [81]. Notably, RNase-7 and RNase-5 have emerged as being especially important in acting as AMPs in the skin.
RNase-7 is a 14.5 kD protein that is found in the skin [82]. It is constitutively expressed at a relatively high level in normal skin with the highest levels of expression in the stratum corneum (Fig. 6.3). It may be important in skin disease as greater than two-fold increases of expression is seen in psoriatic skin [16, 83]. It is active against both Gram-positive and Gram-negative organisms, such as Propionibacterium acnes, S. aureus, E. coli, and P. aeruginosa; as well as fungi, such as C. albicans [82]. RNase-7 has also been shown to be bactericidal against multi-resistant bacteria such as methicillin-resistant S. aureus and vancomyin-resistant enterococci [82]. Its relationship with S. aureus is notable as the application of RNase-7-neutralizing antibodies to the surface of the skin increased the growth rate of S. aureus [84], and as RNase-7 gene expression was found to be significantly reduced in S. aureus-positive skin [85].
While the name RNase-7 may suggest antibacterial properties through ribonuclease activity, a study using ribonuclease-inactive recombinant RNase-7 showed no difference in killing activity against P. aeruginosa, E. faecium, and E. coli compared to control [86]. The exact bactericidal mechanism of RNase-7 is still being elucidated. Structurally, RNase-7 contains four disulfide bonds, similar to the defensin family and has been shown to bind and permeabilize bacterial membranes (Fig. 6.1) [87]. While RNase-7 has yet to be demonstrated to be antiviral, it is hypothesized that its ribonuclease activity may have a role against viruses, a hypothesis supported by the observation that RNase-7 expression is upregulated in keratinocytes in response to dengue virus [88]. Immunologically, RNase-7 can be induced by various stimuli including IL-1β, TNF-α, IFNγ [83] UVB [89], S. aureus [84], and P. aeruginosa. RNase-7 is found in relatively high levels of constitutive expression in normal adult skin and in lower levels in prenatal skin [90].
RNase-5 is found in abundant levels in the stratum corneum and also plays an antimicrobial role in the skin [91]. Initially, RNase-5 was named angiogenin because it was first associated with its capacity to induce angiogenesis [92], but it was later observed to share homology with the RNase A superfamily. RNase-5 has been shown to exhibit bactericidal effects against S. pneumonia and limited effects against E. faecalis, L. monocytogenes, MRSA, and P. aeruginosa [91, 93]. This limited killing spectrum compared to RNase-7 is thought to be attributed to the lack of lysine residues required for membrane permeabilization found in RNase-7. RNase-5 also exerts antifungal activity against C. albicans, a property attributed to the inherent ribonucleolytic properties of RNase-5.
Other members of the RNase A family have also been demonstrated to possess antimicrobial and immunological properties. RNase-2, also known as eosinophil-derived neurotoxin, attracts dendritic cells and enhances Th-2 mediated responses [94]. RNase-2 also exhibits antimicrobial activity against S. aureus [93]. RNase-3, also called eosinophil-cationic protein, has been demonstrated to permeabilize membranes independent of its RNA degrading properties, kill parasitic worms, and induce mast cell degranulation. RNase-3 has also been demonstrated to be antibacterial and antiviral.
Other Antimicrobial Peptides
There is growing evidence to support the presence of multiple AMPs in the human skin in addition to those discussed thus far. Discussion of all the different peptides and proteins with antimicrobial activity present within human skin is beyond the scope of this chapter. However, we would like to highlight a few additional AMPs found within the skin that have recently gained more attention. A summary of some of these AMPs is laid out in Table 6.3.
Table 6.3
Additional mammalian antimicrobial peptides with relevance to skin
Cell type | Comments | |
|---|---|---|
Adrenomedullin | Blood Skin Mucosal secretions | Regulation of skin growth and wound repair Active against E. coli and S. aureus |
α-melanocyte stimulating hormone (α-MSH) | Keratinocytes | Active against S. aureus and C. albicans; inhibits HIV-1 replication |
Calgranulin A/B | Keratinocytes | Inhibits growth of C. albicans |
Connective tissue activating peptide 3 (CTAP-3) | Platelets | Microbicidal for bacteria >fungi |
Elafin | Keratinocytes | Active against P. aeruginosa |
Fibrinopeptide A (FP-A) | Platelets | Microbicidal for bacteria >fungi |
Fibrinopeptide B (FP-B) | ||
Lactoferrin | Keratinocytes Neutrophils | Active against Gram-negative bacteria, Decreasing IL-1, IL-2 and TNF-α Enhancing monocyte and NK cell cytotoxicity |
Lysozyme | Keratinocytes | Active against Gram-positive and some Gram-negative |
Neuropeptide Y | Langerhans cells | Broad spectrum |
Neutrophil gelatinase-associated lipocalin (NGAL) | Infiltrating neutrophils | Bacteriostatic; mechanism of action based on iron sequestration |
P-cystatin α | Keratinocytes | Inhibits growth of S. aureus
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|