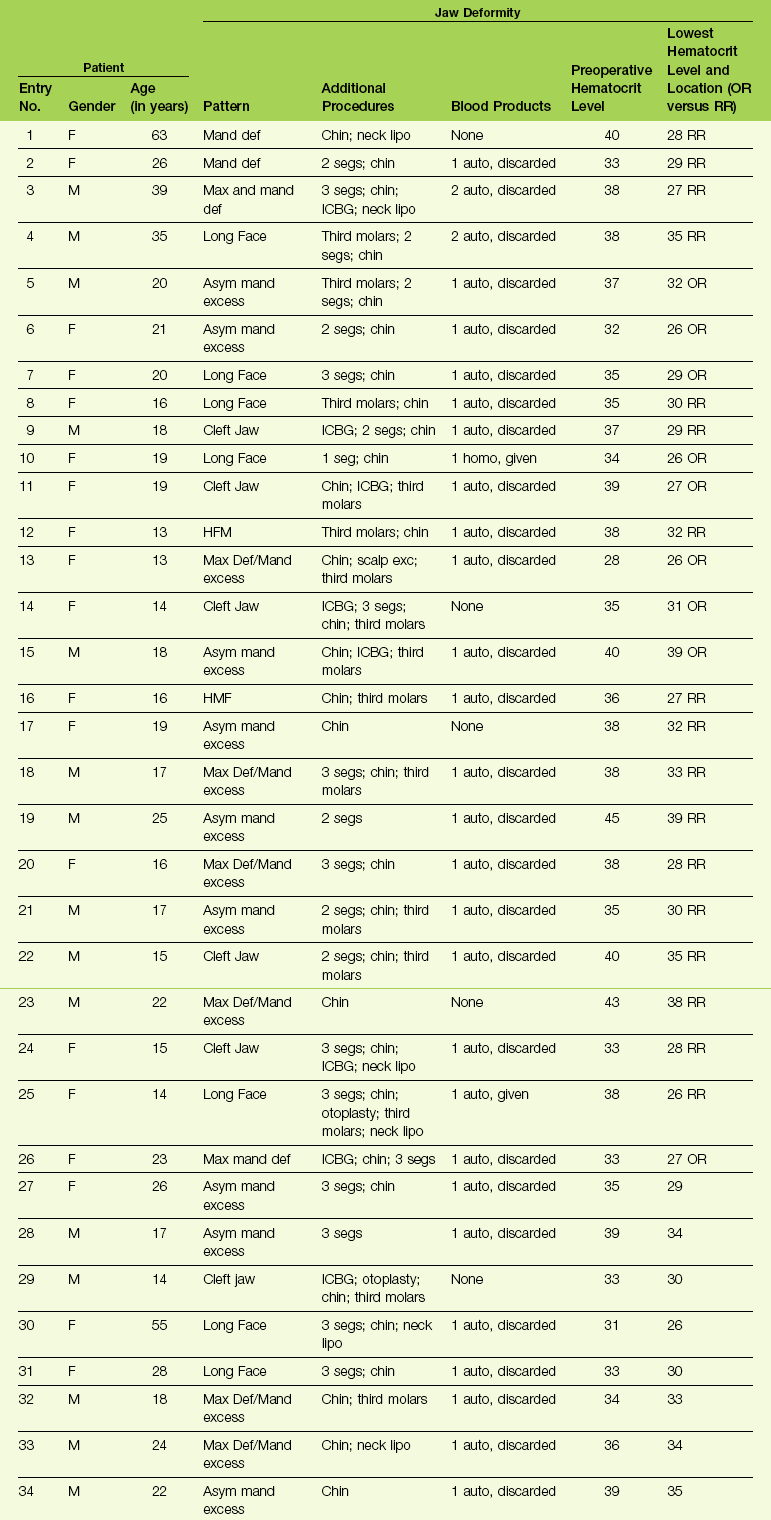
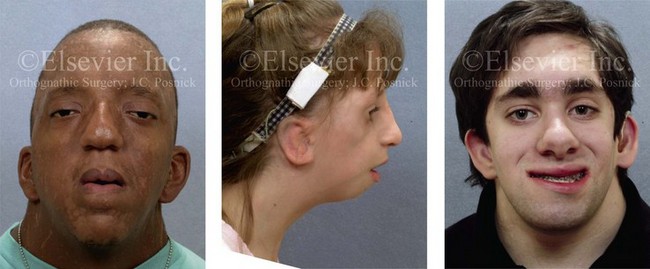
11 • Use of Deliberate Hypotensive Anesthesia to Limit Blood Loss • Use of Hemorrhage Depressors to Limit Blood Loss • Deliberate Hypotensive Anesthesia Used in Orthognathic Surgery: Review of the Literature • The Need for Blood Replacement in Orthognathic Surgery • Perioperative Airway Management in the Patient with a Dentofacial Deformity • In-Hospital Convalescence After Orthognathic Surgery • At-Home Convalescence and Outpatient Care After Orthognathic Surgery Deliberate hypotensive anesthesia (DHA) to provide a less bloody field and better operative conditions was first proposed by Harvey Cushing in 1917 for neurosurgical procedures.58 Since then, a spectrum of pharmacologic agents (e.g., nitroglycerine, calcium-channel blocking agents, short-acting beta-adrenergic blockers, purine compounds) and techniques have been used in a variety of combinations in an attempt to accomplish the same objectives.* In 1950, Enderby and colleagues demonstrated the value of DHA to reduce blood loss in a series of 35 patients.78–81 Eighteen of the 35 (51%) were judged to have an excellent reduction in blood loss during deliberate hypotension, and 8 of the 35 (23%) were judged to have a moderate reduction. The authors attributed this inconsistent effectiveness to individual vascular responses to the hypotensive drugs that were used. They surmised that bleeding at the surgical site could be further minimized if the wound itself was kept uppermost (rather than dependent) through the use of operating room table positioning (e.g., reverse Trendelenburg position for head and neck procedures). Through special positioning, arterial vessels in the operative field would have less pressure, veins would drain more easily, and thus bleeding at the surgical site should be less. One of the earliest studies to quantify reductions in blood loss with DHA was performed by Eckenhoff and Rich, who completed research in patients who were undergoing a variety of operations, including rhinoplasty, portacaval shunt, and craniotomy for aneurysm.72,73 Half of the patients underwent DHA (n = 115), and the other half (n = 116) went without hypotension. For each of the procedures, blood loss decreased by 50% or more with hypotension techniques. This was the first DHA study to measure blood loss with the use of a control group. By doing so, it greatly added to the power of the research confirming the value of DHA. Evidence supporting the use of DHA to reduce blood loss has most commonly been found in studies of orthopedic procedures. Thompson and colleagues studied hypotensive anesthesia to reduce blood loss during total hip arthroplasty.255 The mean arterial pressure (MAP) of the patients (n = 30) was reduced to 50 mm Hg. The techniques used to do this included sodium nitroprusside (n = 12) and high inspired concentrations of halothane (n = 9); a control group of patients (n = 9) was normotensive. Blood loss was 1200 mL for the normotensive control subjects but only 400 mL for both hypotensive groups. No anesthesia-specific complications were seen. In 1979, Eerola and colleagues completed a controlled study of 55 patients who were undergoing total hip arthroplasty.76 They found that the 38 patients who were given pentolinium tartrate and halothane for DHA had less blood loss. That same year, Vazeery and colleagues studied patients who were undergoing total hip replacement (n = 25). The patients were given sodium nitroprusside to lower arterial blood pressure.262 They had significantly less blood loss than the control group (n = 25), which was not undergoing deliberate hypotension. The value of DHA to reduce blood loss has also been confirmed for other procedures, including craniotomy, middle-ear surgery, radical cancer operations, and a variety of head and neck surgeries. Didier and colleagues suggested that the depression of cardiac output correlated better with a dry field than did arterial blood pressure.66 To determine whether a decrease in MAP or cardiac output was the primary cause of the decreased blood loss, Sivarajan and colleagues studied “healthy” (American Society of Anesthesiologist I) subjects (n = 20) who were undergoing bilateral sagittal split ramus osteotomies of the mandible.239 Cardiac output decreased 37% with the use of trimethaphan, but it decreased only 27% with sodium nitroprusside. Interestingly, blood loss was similar for both groups, although cardiac output was two times greater with nitroprusside. The authors concluded that blood pressure rather than cardiac output was the primary determinant of how much blood was lost. Clearly, one can decrease MAP by increasing the amount of inhaled anesthetics such as sevoflurane, isoflurane, and desflurane. Certain drugs that permit moment-to-moment controlled blood pressure are the most popular. The differences in pharmacologic properties among these agents suggest that combinations of these drugs may provide a better pharmacologic profile than could be provided by any agent used alone.234 Inadequate oxygen supply to the brain and myocardium as a result of inadequate tissue blood flow or perfusion pressure is the principal hazard of DHA, particularly with regard to the effects on cerebral metabolic homeostasis.* Studies to date have shown that the responsible use of DHA does not produce permanent changes in cerebral hemodynamics. For example, the cognitive performance of elderly patients on psychological tests given several days after total hip arthroplasty that involved DHA did not differ from their performance before surgery. The current clinical rationale for studying a lower limit for MAP (e.g., 50 mm Hg to 55 mm Hg) in normotensive patients is based on the belief that this range represents the lowest MAP at which autoregulation of the cerebral blood flow is still intact. However, for chronically hypertensive patients or for patients who are older, the curve is thought to shift to the right. In other words, a higher MAP is required in these individuals to maintain autoregulation. The partial pressure of carbon dioxide in the blood (paCO2) is also an important consideration during DHA. This is because cerebral blood flow changes linearly with paCO2. Whether one drug is better than another for preserving cerebral blood flow is debated. The preservation of cardiac function is essential, but it is known to be altered by DHA techniques.† Interestingly, cardiac function is better preserved with isoflurane than with equal hypotensive doses of halothane. Studies have shown that isoflurane is suitable for healthy patients who are undergoing DHA. Clinical studies also show that the cardiovascular effects of general anesthetic doses of propofol are similar to those of isoflurane. During deliberate hypotension, the maintenance of an oxygen supply that is sufficient for the metabolic needs of the myocardium is of primary importance. As a general rule, patients with known or suspected ischemic heart disease should not undergo DHA. Renal blood flow normally equals 20% to 25% of cardiac output. Renal circulation is greatly influenced by autoregulatory mechanisms.61,110,159 Clinical studies suggests that normovolemic patients typically experience the rapid recovery of urine production after the discontinuation of DHA.255 Thompson and colleagues could find no significant changes in serum creatinine, blood urea nitrogen, or serum or urinary electrolytes for patients (n = 30) who had undergone DHA.255 The precise incidence of complications with the use of DHA is difficult to determine.19,75,41,152,179,248,253 Current data regarding non-fatal complications indicate that hypotension of 50 mm Hg to 65 mm Hg is not a risk factor for young healthy patients (American Society of Anesthesiologists [ASA] 1 and 2). Alternatively, for those individuals with underlying organ dysfunction, the use of DHA may put them at increased risk. Thus, all potential candidates should undergo a complete history and physical examination before surgery. The decision to use DHA should not be made without careful preoperative consideration of patient-specific risk factors. Before beginning an orthognathic procedure, the logistics of patient positioning, the expected length of the surgery, and the confirmation of patient-specific risk factors for DHA should be discussed by the anesthesiologist and the surgeon.264 Better drugs, more accurate monitoring, a greater level of experience with the techniques, and the consistency of the surgical procedures themselves have permitted a higher percentage of patients to benefit from DHA.* Nevertheless, relative contraindications include a history of cerebrovascular or cardiovascular disease, renal dysfunction, liver dysfunction, peripheral vascular disease, and severe anemia. Fortunately, the orthognathic patient rarely suffers from these dysfunctions. Exceptions include older individuals who are undergoing orthognathic surgery for the treatment of obstructive sleep apnea.228,261 Given current demographics, this is likely to represent an ever-increasing patient population (see Chapter 26). Tranexamic acid is a synthetic amino acid that inhibits fibrinolysis and that has been shown to reduce blood loss and the need for blood transfusion during specific surgical procedures, including total knee arthroplasty, spine surgery, cardiac surgery, and orthognathic surgery.* It has also been used for the treatment of postoperative bleeding in anticoagulated patients after oral surgery (e.g., the extraction of teeth).32,212 Senghore and Harris showed that a single intravenous preoperative dose of tranexamic acid was effective for preventing excessive postoperative bleeding in healthy adult patients who were undergoing third molar extraction.232 Choi and colleagues completed a double-blind, randomized, controlled trial to study the effects of tranexamic acid on blood loss during orthognathic surgery.51 This trial included 73 consecutive patients who were undergoing bimaxillary orthognathic osteotomies. Each study patient received either a bolus of tranexamic acid (20 mg/kg) or a placebo (normal saline) intravenously just before surgery. All patients underwent DHA in accordance with standard hospital protocol. Intraoperative blood loss, operative time, the transfusion of blood products, and perioperative hemoglobin and hematocrit levels were recorded. The total blood loss and the blood loss during the maxillary (Le Fort I) procedure were reduced significantly in the group that received tranexamic acid as compared with the control group. The difference in blood loss during maxillary surgery was 428 mL versus 643 mL. The difference in total blood loss was 878 cc versus 1257 cc. The authors concluded that the use of a preoperative intravenous bolus of tranexamic acid (20 mg/kg) reduces blood loss as compared with a placebo during bimaxillary orthognathic osteotomies. Desmopressin acetate is an analog to the naturally occurring hormone vasopressin.4,114,115,138,147,222,225,245 It enhances the vascular endothelial cell release of a number of compounds, including high multimetric von Willebrand factor, factor 7c, factor 7ag, and tissue-type plasminogen activator. Desmopressin by itself has been suggested to be the factor that is responsible for the increased hemostatic activity seen in patients with liver dysfunction and uremia. Additional observations indicate that desmopressin may improve platelet function. Both of these hemorrhage depressors—desmopressin and tranexamic acid—have been used in combination with DHA during orthognathic surgery at the University of Göteborg Hospital since 1999. Zellin and colleagues retrospectively evaluated and compared the total perioperative blood loss for a group of patients who were assigned to either DHA or a combination of DHA and the use of both of these hemorrhage depressors (i.e., desmopressin and tranexamic acid).276 Thirty orthognathic patients who were consecutively operated on via a standardized Le Fort I osteotomy were assigned to either the control group (n = 15) or the treatment group (n = 15). Both groups underwent DHA. The treatment group received additional hemorrhagic depressors (i.e., both tranexamic acid and desmopressin). The mean blood loss was 740 cc in the control group and 400 cc in the treatment group. This was a statistically significant reduction in blood loss in the treatment group (1 g tranexamic acid intravenously and 0.3 µg per kilogram of body weight of desmopressin subcutaneously). Schaberg and colleagues were the first to evaluate blood loss and the human physiologic response with the use of DHA in the form of sodium nitroprusside during orthognathic surgery.227 They studied a consecutive series of ASA I patients undergoing Le Fort I maxillary osteotomies. Each was placed in reverse Trendelenburg position (10 degrees), and sodium nitroprusside was used to induce hypotension to not less than 80 mm Hg systolic pressure or 60 mm Hg MAP. The mean estimated blood loss was 256 ± 35 mL. No control group was used for comparison. Washburn described a retrospective series of 58 individuals who underwent maxillofacial procedures (n = 53 orthognathic patients) in which morphine was used to supplement halothane to induce hypotension.267 The goals were to limit blood loss and to improve the surgical field. There was one death (possibly from halothane-induced liver failure), and only one patient required blood replacement. Golia and colleagues studied nine patients who underwent either maxillary or combined maxillary and mandibular osteotomies; the DHA techniques that were used included halothane, nitrous oxide, and supplemental doses of muscle relaxants and narcotics.106 Nitroglycerin was added for the purpose of lowering the MAP during periods with a high potential for increased blood loss (e.g., Le Fort I down-fracture). Estimated blood loss averaged 439 mL and the hemoglobin/hematocrit level fell from 13.8 hemoglobin/41 hematocrit to 12 hemoglobin/36 hematocrit (4.9%). The authors concluded that nitroglycerin was a safe and efficacious agent to help control MAP and blood loss in the surgical field during maxillary and mandibular osteotomies. No control group was used. McNulty and colleagues completed a study of 12 patients who underwent orthognathic procedures (i.e., Le Fort I osteotomy or sagittal split ramus osteotomy of the mandible) under DHA.169 The anesthesia agents that were used included fentanyl, isoflurane, and bolus doses (i.e., 10 mg to 20 mg) of labetalol to control the blood pressure in an attempt to improve the surgical field and limit blood loss. The authors reported clinical satisfaction with the surgical field and no complications. No control group was used. Blau and colleagues compared esmolol in 15 patients with sodium nitroprusside in 15 other patients as the primary drugs for DHA to limit blood loss during Le Fort I osteotomy.31 All study patients were physically rated as ASA I or II. The mean MAP averaged 58 mm Hg to 60 mm Hg, with a target range set at 55 mm Hg to 65 mm Hg. The mean heart rate was maintained at approximately 70 beats/min with esmolol and 100 beats/min with nitroprusside. The surgeons generally rated bleeding as mild to moderate and the surgical field as “drier” with esmolol. The total measured blood loss was less with esmolol (i.e., 436 mL with esmolol versus 895 mL with nitroprusside). The authors found the advantages of esmolol as compared with nitroprusside to include greater control of the blood pressure, the reduction of blood loss, and a drier surgical field. Precious and colleagues studied 50 orthognathic surgery patients in a prospective, randomized, blocked, stratified, and single-blind fashion.206 All patients underwent either sagittal split ramus osteotomies of the mandible, Le Fort I osteotomy, or genioplasty. One group of patients (n = 25) received DHA, whereas the other group (n = 25) received anesthesia with no specific attempt to reduce blood pressure during the operation. The surgeon, who was unaware of which group the patient had been assigned to, rated the surgical field every 15 minutes. At the completion of surgery, three different methods were used to estimate or calculate blood loss. The duration of the procedure was recorded from the time of the first incision to the time of the last stitch placement. The estimated blood loss was significantly less when DHA was used. The surgical field was also rated better, but there was no significant difference in the duration of the procedure with or without the use of DHA. The authors concluded that DHA results in both reduced blood loss and improved visibility in the surgical field. Enlund and colleagues studied 36 patients who were undergoing orthognathic surgery.84 Each patient was randomly assigned to be either hypotensive (n = 18) or normotensive (n = 18) during surgery. Hypotension was achieved with the use of isoflurane to a MAP of 50 mm Hg to 64 mm Hg. The hypotensive group had significantly less blood loss per minute. Only two of the patients in each group underwent bimaxillary osteotomies. A majority of the patients in each group underwent only mandibular ramus osteotomies. None of the patients in either group required blood replacement. Dolman and colleagues completed a prospective, randomized, blinded-to-the-surgeon clinical trial of DHA in a consecutive series of orthognathic patients.69 The purpose of the study was to compare the quality of the surgical field, the blood loss, and the operative time with either hypotensive or normotensive anesthesia during Le Fort I osteotomy. The study patients (n = 23) were randomized into normotensive or hypotensive anesthesia treatment groups. No local anesthesia with a vasoconstrictor was used by the surgeon. In the experimental group, hypotensive anesthesia was maintained with propofol (3 mg/kg/hr), sufentanyl (0.5 mg/kg/hr), and isoflurane (0.5% in 100% oxygen). The quality of the surgical field in both groups was assessed intraoperatively by direct observation and again postoperatively with video imaging. The surgical time was measured on the videotape, and blood loss was measured by volumetric and gravimetric techniques. There was a statistically significant correlation between the surgeon’s perception of the quality of the surgical field and the blood pressure. There was a statistically significant reduction in blood loss when using hypotensive anesthesia. However, there was no significant reduction in operative time when using hypotensive anesthesia. The authors concluded that hypotensive anesthesia is a valuable technique for reducing blood loss and improving the quality of the surgical field during Le Fort I osteotomy. Praveen and colleagues completed a prospective randomized clinical study to assess DHA and blood loss during orthognathic surgery.204 A total of 53 patients who were undergoing orthognathic surgery were included in the study. The patient ages ranged from 15 to 33 years. They were randomly allocated to have either normotensive anesthesia or to be given DHA. Median blood loss with DHA was 200 mL; under normotensive anesthesia, it was 350 mL. The authors concluded that there was a pronounced reduction in blood loss during orthognathic operations with the patient under DHA as compared with normotensive anesthesia. Manola and colleagues completed a prospective randomized comparison of three types of pharmacologic agents to induce hypotension during functional endoscopic sinus surgery.165 The patients were divided into three equal groups in accordance with the agents used: 1) sufentanyl/sevoflurane; 2) remifentanil/propofol; and 3) fentanyl/isoflurane. Evaluation included surgical field rating and measured blood loss. The authors found that the quantity of blood loss and the visibility of the surgical field were improved with the use of either remifentanil and sufentanyl as compared with fentanyl. Choi and colleagues completed a comparative study to report about the use of three different pharmacologic drugs to induce DHA in patients who were undergoing orthognathic surgery (i.e., Le Fort I and sagittal split ramus osteotomies of the mandible).49 The 50 adult patients were randomly allocated to receive either nitroglycerin (n = 25) or remifentanil (n = 25). The MAP and the heart rate were measured at intervals. The heart rates and blood pressures were significantly better controlled with the use of remifentanil as compared with nitroglycerin. Interestingly, there were no significant differences in the surgical field ratings or the measured blood loss between the two groups. Farah and colleagues completed a comparative study to report on the use of two different pharmacologic protocols to induce DHA in patients submitted to orthognathic surgery.90 Twenty ASA 1 patients between the ages of 17 and 44 years were randomly assigned into two groups: group 1 (clonidine and remifentanil) and group 2 (dexmedetomidine and isoflurane). Other drugs that were used as part of the anesthesia regimen were common to both groups. Specific patient parameters were assessed, including arterial blood pressure; heart rate; temperature during the intraoperative and postoperative periods; incidence of nausea and vomiting; postoperative pain; awakening time; extubation time; and postanesthesia recovery time. The results of the study showed that there were no significant differences between the two groups with respect to physiologic responses or surgical time. Both study protocols proved to be effective and safe. Each was suggested as a satisfactory option to achieve DHA during orthognathic procedures when there is an expectation of significant blood loss. For individuals who are undergoing complex orthognathic surgery (i.e., Le Fort I and sagittal split ramus osteotomies of the mandible)—often in combination with other simultaneous procedures (e.g., genioplasty, liposuction, septoplasty, inferior turbinate reduction, removal of wisdom teeth)—a preoperative doctor–patient discussion of the need for intraoperative blood replacement is not just an academic issue.92,103,107,149,163,201 Published studies indicate that individuals who are undergoing bimaxillary surgery require blood replacement in the range of 2.5% to 75%.15,64,138,142,148,156,200,218,223,226,235,259,274 Rummasak and colleagues retrospectively studied patients who underwent bimaxillary osteotomies (n = 208) during a 4-year timeframe from 2005 to 2009 at a single institution. The authors reviewed possible factors for intraoperative blood loss and looked for statistical significance. They documented that, with consistent anesthesia techniques, the two factors of greatest importance for blood loss were 1) the experience of the surgeon and 2) the overall operative time.223 Madsen and colleagues conducted a prospective study to evaluate the predictive value of the viscoelastic properties of whole blood samples collected preoperatively in relation to intraoperative blood loss in patients who were undergoing orthognathic surgery (n = 41). They concluded that, with other variables controlled for, the etiology of intraoperative bleeding can be predicted by means of preoperative thromboelastography. This is a global method that addresses the complex interplay among coagulation factors, blood platelets, and components of the fibrinolytic system. Blood platelet count, activated partial thromboplastin time, prothrombin time, plasma fibrinogen concentration, and D-dimer concentration were determined by routine methods. The authors conclude that thromboelastography results in individual patients can be used for the evaluation of bleeding risk. Further studies will be required to confirm the validity of their findings.162 Methods to limit the need for blood replacement continue to evolve and include refinements in DHA techniques as well as in surgical management (i.e., patient selection, collaborative clinical efforts, and the efficiency of the operation and the postoperative care).102 Despite stringent donor screening criteria and the rigorous testing of every unit of blood, there remains a risk for the transmission of several pathogens.70,93,104,108,134,177 For viruses such as the human immunodeficiency virus (1 in 2,135,000), hepatitis B virus (1 in 205,000), and hepatitis C virus (1 in 2,000,000), this is due mainly to “window-period” donations.34,247 The risks of newly emerging pathogens that can affect blood safety (e.g., Creutzfeldt-Jakob disease) remain unclear. There are other pathogens (e.g., cytomegalovirus, parvovirus B19) that are common in the general donor population and that may pose a risk in the immunosuppressed patient.213 In addition, allogenic transfusion has been shown quantitatively to be an important contributor to postoperative infection.94,260 The options of autogenous and donor-directed blood banking and their popularity for elective surgery are attributed to their perceived safety, to refinements in blood bank administration, and to public concern about the hazards of allogenic blood replacement.16,86,91,105,118,167,184,210,240 In at least one state (California) and one nation (Germany), the preoperative discussion of blood replacement options is mandated by law and must be documented in the medical record. Other alternatives to allogenic transfusion include acute normovolemic hemodilution, intraoperative blood salvage, and perioperative therapy with recombinant human erythropoietin (Procrit).35,101,136,170,173,180,230,231 The absolute need for and actual use of blood transfusion in the practice of orthognathic surgery remains dependent on considerations that relate to total whole body oxygen delivery and its ability to meet the body’s metabolic needs.56,121,175,189 Many individual hospitals have instituted strict criteria for the transfusion of blood products.215 Although mandated criteria may decrease the use of blood transfusions, it remains unclear if complications or delays in recovery have occurred as a result.250 Posnick and colleagues completed a retrospective study to review blood replacement practices in a consecutive series of a single surgeon’s experience with patients who all underwent, at a minimum, simultaneous Le Fort I maxillary osteotomy, bilateral sagittal split ramus osteotomies of the mandible, septoplasty, and inferior turbinate reduction procedures.202 Many patients also underwent additional adjunctive procedures. A chart review of a consecutive series of the surgeon’s patients (n = 34) who underwent elective bimaxillary orthognathic surgery (Le Fort I osteotomy and sagittal split osteotomies of the mandible) in combination with intranasal (septoplasty and inferior turbinate reduction) and often other simultaneous procedures during a 5-month time frame at a single institution was carried out (see Table 11-1). The chart review occurred at least 3 months after surgery for all patients. TABLE 11-1 Blood Replacement for Complex Orthognathic Surgery From Posnick JC, Rabinovich A, Richardson DT: Blood replacement for complex orthognathic surgery, J Oral Maxillofac Surg 68:54–59, 2010. Each patient was also evaluated for complications specific to the procedures that were carried out. Potential complications specific to the orthognathic procedures included infection that required the extended use of antibiotics or drainage procedures; dental injury; fibrous union; aseptic necrosis; bleeding that required secondary treatment; and oroantral, oronasal, and orocutaneous fistulas. Potential complications specific to the intranasal procedures included postoperative nasal bleeding that required packing or cauterization; the need for postoperative blood transfusion specific to nasal bleeding; the presence of septal perforation; and postoperative nasal obstruction that required additional procedures within 3 months after orthognathic surgery. Potential perioperative airway compromise was also reviewed, including the need for delayed extubation, reintubation, or tracheotomy. Potential complications specific to transfusions, including infections and autoimmune reactions, were also sought. The patient records that were reviewed included the office chart; the hospital records; and the data stored at the Red Cross (hospital) blood bank. Specific study data collected for each patient (n = 34) can be found in Table 11-1. The current recommendation of the National Heart, Lung and Blood Institute’s conference report is that autogenous blood donations are indicated for patients who are having surgical procedures for which blood is usually cross-matched.185 The less likely the transfusion for a specific procedure, the more likely that donated blood will not be used. The recommendations are that patients should not be encouraged to donate blood for autogenous use during surgery unless there is a greater than 10% likelihood that they will need it. The historic literature indicates a wide range (i.e., 2.5% to 75%) with regard to the use of transfusion during bimaxillary orthognathic surgery. The review study performed by Posnick and colleagues indicated only a 6% need.202 In current practice, the need for blood replacement during bimaxillary orthognathic surgery may be closer to 2%. The high-risk individual (e.g., an adult with obstructive sleep apnea and other comorbidities) can usually be recognized from the outset, and preparations can be made in advance (i.e., blood typing and cross-matching). Another common question is, “Should the indications for the use of autogenous blood transfusion differ from those for the use of allogenic blood transfusion?” If precise indications for a blood transfusion could be defined, autogenous blood would not be given more frequently than allogenic. Although the benefits of allogenic and autogenous blood transfusions are the same, the risks are not. Therefore, the risk-to-benefit ratio clearly supports the more liberal use of autogenous blood. It has been documented that patients who preoperatively donate blood are more likely to be transfused earlier and more frequently than patients who do not auto-donate. Studies have challenged the use of autologous blood donations for elective surgery, because there is the possibility of blood overcollection, the wasting of autologous units, and the costs of preparation and storage. Cohen developed an interesting mathematical model to analyze the use of preoperative autogenous blood donation.53 The model clarifies how clinicians can customize autogenous blood collection for individual patients to minimize waste (i.e., the need to discard non-transfused blood). A basic airway decision for the surgeon and the anesthesiologist to make for the individual who is to undergo orthognathic surgery and who therefore requires general anesthesia is whether to use induced anesthesia and then directly intubate with or without the use of a Glide Scope (Bell Medical Inc., St. Louis, MO); to use awake fiber-optic intubation; or to use a percutaneous technique (e.g., cricothyroidotomy and tracheostomy). The patient with a dentofacial deformity who presents for general anesthesia should be considered to have a difficult airway until it is proven otherwise (Fig. 11-1).45,48,123,127,141,146,172,203,207,217 Figure 11-1 Perioperative risk to the airway and its management should be considered during all phases of orthognathic surgery, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation. Individuals who are scheduled for orthognathic surgery who have been recognized to be at high risk for airway compromise should be discussed in advance with the anesthesia team to arrange for special needs. Three patient examples in which these issues came into play are shown: an adult with Noonan syndrome; a teenage girl with Treacher Collins syndrome; and a teenager with the Klippel–Feil anomaly. Each of these patients also has the potential for cervical spine anomalies with limited neck range of motion. The Risk of spinal cord injury should also be evaluated before orthognathic surgery. The head and neck examination of a patient who is scheduled to undergo endotracheal intubation should include his or her mouth-opening ability; the ability to protrude the lower jaw; any limitations of neck extension; the measurement of the thyromental distance; and the Mallampati test.140,164,165 These parameters cover the standard reference points to determine whether or not to expect a difficult airway. These parameters represent components of the basic head and neck airway evaluation recommended in the guidelines of the American Society of Anesthesiologists.6 1. Mouth-opening ability. This is measured as the interincisor distance. A value of less than 4 cm has been proposed as an indicator of possible difficult intubation. Fibrous or bony temporomandibular joint ankylosis will absolutely diminish mouth opening. Muscle spasms or pain may also limit mouth opening, but these factors can be overcome with muscle relaxants and analgesics. 2. The inability of the individual to protrude the lower jaw forward. When the mandibular incisors cannot protrude in front of the maxillary incisors, this is considered to be an indicator of a difficult intubation. It is often an indirect test for mandibular retrognathia. In addition, it can be a deceiving indicator of normal jaw morphology in the individual with a normal mandible but with maxillary deficiency. 3. Limited neck extension. An angle of less than 20 degrees as measured between the occlusal surface of the maxillary teeth and the neck when it is in full extension is felt to be suggestive of a difficult direct laryngoscopy. A percentage of dentofacial deformity patients will have cervical spine anomalies that prevent adequate and safe neck extension. 4. The Mallampati test. The visibility of the pharyngeal structures when the mouth is wide open is considered to be of limited use as an individual assessment. However, when this is considered in combination with other physical findings, it is of some value. The dentofacial deformity patient with baseline obstructive sleep apnea is likely to have a Class IV Mallampati classification. The Mallampati classification is determined by having the awake patient in the sitting position open his or her mouth widely and then protrude the tongue completely forward. The clinician then documents which structures can be visualized. Class I: The soft palate, the fauces, the entire uvula, and the pillars are visualized. Class II: The soft palate, the fauces, and a portion of the uvula are visualized. Class III: The soft palate and the base of the uvula are visualized. 5. The thyromental distance. This is often used as an indirect gauge of mandibular morphology. The ratio of a patient’s overall height to his or her thyromental distance may be used as an indirect assessment of mandibular deficiency. • The status of the teeth (e.g., mobility, missing teeth, removable and fixed appliances, general oral heath); • The neck circumference (this is an indirect indicator of obstructive sleep apnea) • Any presenting dentofacial deformity (e.g., maxillo-mandibular deficiency) • Any anatomic cause of nasal airway obstruction (e.g., septal deviation, posterior choanal atresia, enlarged turbinates) 1. The maintenance of oxygenation is essential. Preoxygenation is performed before the induction of anesthesia. Mask ventilation is also used in between attempts at tracheal intubation. 2. Trauma to the upper airway must be prevented. The first attempt at tracheal intubation should be performed under optimal conditions (i.e., patient positioning, preoxygenation, and equipment preparation). 3. The anesthesiologist should have a backup plan in place before initiation of the primary technique. He or she must have the skills and equipment needed to execute the backup plan for the patient with a difficult airway. The recent introduction of video-assisted laryngoscopy instruments have lessened the need for more invasive methods to secure the airway, such as the use of a fiber-optic bronchoscope and percutaneous techniques. The backup plan should include the eventuality that, if noninvasive techniques do not restore oxygenation, then cricothyroidotomy (which is generally the percutaneous airway method of choice) is ready to go. In an actual “cannot intubate—cannot ventilate” situation, immediate percutaneous access to the cricothyroid membrane is essential. 4. The anesthesiologist should seek the best help available as soon as difficulty with tracheal intubation is experienced. He or she should be aware of available backup personnel or colleagues who can be quickly called in to assist during an emergency. The practical guidelines for the management of the difficult airway have been reported by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway.6 The guidelines recommend the following: 1) a detailed evaluation of the airway; 2) a general physical examination; 3) additional patient-specific evaluations, as needed; 4) basic preparation for difficult airway management; 5) a strategy for the intubation of the difficult airway; 6) a strategy for the extubation of the difficult airway; and 7) arranging for follow-up care. 1. An assessment of the likelihood and anticipated clinical impact of four basic problems that may occur alone or in combination: 2. A consideration of the relative clinical merits and feasibility of the three basic airway management choices:
Anesthesia Techniques, Blood Loss/Fluid Replacement, Airway Management & Convalescence in the Treatment of Dentofacial Deformities
Use of Deliberate Hypotensive Anesthesia to Limit Blood Loss
Historical Perspective
Techniques to Induce Deliberate Hypotensive Anesthesia
Effects of Deliberate Hypotensive Anesthesia on Organ Function
Complications Associated with Deliberate Hypotensive Anesthesia
Special Considerations for the Orthognathic Patient
Use of Hemorrhage Depressors to Limit Blood Loss
Tranexamic Acids
Desmopressin
Deliberate Hypotensive Anesthesia Used in Orthognathic Surgery: Review of the Literature
The Need for Blood Replacement in Orthognathic Surgery
Background
Review of Study
Controversies in Blood Replacement for Orthognathic Surgery
Perioperative Airway Management in the Patient with a Dentofacial Deformity
Head and Neck Examination
Prevention of Complications during Tracheal Intubation







