!DOCTYPE html>
6. Silicones and Autoimmunity
Keywords
AllopurinolAntisilicone antibodiesASIA syndromeAutoimmune pathologyEosinophilic fasciitisEtanerceptForeign body reactionImiquimodOral corticosteroidsImmunological reactionSilicones and autoimmunityStill’s diseaseSyndrome of the Gulf WarSjögren’s syndromeTacrolimusTetracyclineIntroduction
Connective tissue diseases have autoimmunity as a common underlying mechanism. This is expressed in clinical manifestations of various characteristics and severity and in the laboratory in the form of autoantibodies. They develop at the moment that environmental factors act on genetically susceptible individuals.
Recent research on genetic susceptibility has shown that environmental triggers frequently act on cellular pathways that contain polymorphisms associated with the disease. When tolerance is broken, the initiating tissue – including dendritic cells – provides a decisive microenvironment that affects cellular immune differentiation and leads to the activation of adaptive immunity. Interferon type 1, produced by innate immune cells, plays a central role in systemic autoimmunity and activates B and T cells. In turn, autoantibodies derived from B cells stimulate dendritic cells to produce interferon type 1. This closes a circle that includes both the innate and adaptive systems [1].
The environmental risk factors for different autoimmune diseases are difficult to assess, due to the limited amount of validated exposure biomarkers on one hand and the rarity of the given autoimmune disease on the other. However, case reports and case series, in place of formal epidemiological data, have identified types of disease among certain occupational groups, as well as an association with a variety of environmental agents, such as crystalline silica, polychloride vinyl, rapeseed oil, drugs, and vaccines.
Silicone is a synthetic polymer considered biologically inert. It is used in a multitude of medical products, among which the most publicly recognized are breast implants. Silicone breast implants have been in use since the 1960s for aesthetic and reconstructive purposes, and reports of their association with autoimmune diseases – including systemic sclerosis, systemic lupus erythematosus (SLE), and rheumatoid arthritis, among others – began to appear in the medical literature soon afterward. As a conceptually new and “atypical” disease, which does not meet established diagnostic criteria for any known connective tissue disease, it has been widely studied [2].
Silicone implants also have been implicated as playing an important role in a new syndrome that encompasses a wide range of manifestations related to immunity, called autoimmune syndrome induced by adjuvants (ASIA). The name “siliconosis” has been adopted for the various manifestations that occur after exposure to silicone. Within this syndrome, it is believed that disease appears after immune adjuvant activity stimulated by various substances, like infectious agents, aluminum salts, and silicone. It seems that activation of the immune system by natural or pharmaceutical adjuvants, as is the case of silicone, can, under certain conditions, be the promotor of an autoimmune response in a genetically susceptible person.
In addition to the local activation of the immune system, silicone can cause systemic effects via the degradation of its fragments, which are not inert and can be transmitted throughout the body, leading, in the long term, to a neoplastic process or, in the short time, to an autoimmune phenomenon [3].
In patients with severe immune reactions triggered by implanted silicone devices, it has been found that there is increased immunoglobulin G in the surrounding tissue and higher levels of anti-silicone antibodies relative to patients with asymptomatic implants. Direct visualization by immunofluorescence also reveals anti-silicone antibodies in the capsular tissue of the implants. In addition, in one study, silicone antiserum antibodies were more frequently detected in implant patients than in controls, as were significantly higher antibody titers after implant rupture. Therefore, one action of silicone or its compounds as an adjuvant can be inferred from the association between breast implants and autoimmunity [4].
The remainder of this chapter discusses some of the connective tissue diseases that might be linked to silicones.
Systemic Sclerosis
Systemic sclerosis is an autoimmune disease of connective tissues. The disease’s characteristics include vasomotor dysfunction, which is very common. However, fibroses with consequent atrophy of the skin, subcutaneous tissue, muscles, and internal organs (digestive tract, lungs, heart, kidney, central nervous system) are the most clinically important manifestations, in addition to the various immunological dysfunctions that accompany these findings.
The etiology of systemic sclerosis is unknown, but several environmental agents are associated with its development. This being said, many of these relationships are based upon case reports and have not been studied systematically. Such agents include polyvinyl chloride, crystalline silica (especially in cases of pulmonary silicosis), rapeseed oil (with aniline), and medications like hydralazine and procainamide.
Case reports of women with breast cancer and silicone implants who developed systemic sclerosis began to appear in the medical literature in 1980. By 1998, there were 290 reported cases of women with connective tissue diseases associated with breast implants, many of whom were reported to have systemic sclerosis. As a result of these published cases, several case-control, cohort, and prospective epidemiological studies were orchestrated. The main conclusion drawn from almost all these studies was that the incidence of systemic sclerosis was not higher among women with breast cancer and implants than among women without breast implants [5].
Silicone is considered to be a biologically inert agent and has been used in many different types of medical devices, including tubes, breast prostheses, penile implants, artificial heart valves, intraocular lenses, and ventriculoperitoneal shunts, among others. After a silicone implant, a tissue response occurs, which is generally limited to a mild foreign-body reaction. It is followed by an encapsulation phase, since capsular tissue generally is formed around any nondegradable material that is too large to be phagocytosed by macrophages and too inert to cause any more than a local foreign-body reaction.
There have been reports of antibodies detected against silicone in human serum, and increased levels of anti-silicone antibodies in most of patients; but no relationship has been uncovered between these levels and autoimmune disease [6]. Increased IgG levels detected in medical silicone implants in two patients with ventriculoperitoneal shunts apparently exhibited immune reactions to these shunts [7]. It was claimed that these experimental findings proved that specific immune reactivity to silicone’s polydimethylsiloxane elastomers can be developed in humans. Blanco and Klykken developed an enzyme-linked immunosorbent assay (ELISA) to detect the binding of IgG to silicone and the IgG subtypes in the serum of patients wearing silicone bands and silicone sponges and having intraocular silicone oil. The presence of autoantibodies (rheumatoid factor, antinuclear antibodies [ANA] and anti-Scl 70) and the signs and symptoms of autoimmune disease were also analyzed. Anti-silicone antibodies were detected in 35.7% of the patients with solid silicone and in 83% of those with silicone oil. This increase in IgG was mainly due to IgG1. However, no increase in the signs or symptoms of autoimmune disease was detected [8].
Another study identified a significant increase in anti-silicone antibodies in the capsule, with IgG and IgM antibodies also discovered by capsular immunofluorescence assay in the tissues of patients with silicone implants. The levels of silicone antiserum antibodies were slightly higher in implant patients than in controls, but this increase was not statistically significant. It also has been reported that levels of anti-silicone antibodies are significantly higher in patients with breast cancer and implants [9].
When added to the above-noted data, the microscopic evidence of silicone discovered in other bodily tissues among breast cancer patients with implants, in addition to the silicone compounds identified in the blood and liver of patients with silicone implants, lead to the conclusion that silicone particles, albeit in very small amounts, can disperse throughout the tissues of the body and lead to the production of specific antibodies. This systemic reaction seems to be related to the amount of silicone in the blood, bodily tissues, and capsule. Previously published results argue against great damage being caused by silicone implants but suggest a slightly increased risk of connective tissue diseases among these women.
Several authors have speculated about the likelihood of a relationship between silicone implants and immune system activation and concluded that there is little evidence supporting systemic activation of the immune system in women with breast implants [10]. Clinical relevance lies in the isolated decrease in C3 and C4 levels in women with silicone implants in the absence of other abnormalities, like elevated levels of ANA.
In other studies, several types of autoantibody have been identified as elevated – in fact, 35% of women have such antibodies – so that it has been concluded that, among genetically susceptible people with silicone implants, there could be an increased risk of developing immunopathology [11].
Eosinophilic Fasciitis
Eosinophilia fasciitis is a skin disease similar to scleroderma, which manifests with peripheral blood eosinophilia, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate (ESR). Typical histopathological findings include a chronic inflammatory infiltration, in deep fascia, with lymphocytes, histiocytes, and, occasionally, eosinophils.
Some reports also describe an association between silicone implants and an overlap between eosinophilic fasciitis and morphea. Morphea and other skin conditions are sometimes linked to exposure to chemical compounds, like silicone. One study suggested that patients with this overlap had a 1.4-fold increased risk of refractory fibrosis [12].
Studies have shown that macrophages exposed to silica in vivo can promote fibroblast proliferation and collagen production. Other results indicate that the silicone can penetrate through the semipermeable elastomeric membrane of the implants, into the capsular tissue that surrounds them, and migrate to local ganglia.
In addition, microscopic evidence has been found that silicone is dispersed throughout other tissues in patients with breast implants. Several studies have demonstrated activation of the immune system and the production of autoantibodies in patients with silicone prostheses.
Vasculitis
Epidemiological evidence exists linking environmental exposure to silica, one of the components of silicone, through the lungs and ANCA-associated vasculitis. One study identified an incidence of 5.5% (8 of 145) for silica exposure with renal involvement among ANCA-positive patients. In another study, 5 of 11 ANCA-positive patients who had glomerulonephritis with necrotizing crescents had a history of significant past exposure to silica. And, in a more recent study, ANCA was detected in 17.1% of patients exposed to silica dust.
Recently, a case was reported of vasculitis associated with ANCA and glomerulonephritis with necrotizing crescents after the implantation of a peritoneal ventricular catheter composed of silicone. The authors speculated that the implanted ventricular-peritoneal shunt contained proinflammatory, initially active cytokines that could induce hypergammaglobulinemia and that these, later, led specifically to the activation of ANCA.
In addition to silicone, gel breast implants usually have a silicone elastomer, and amorphous silica is generally used to increase the hardness of the elastomer shell. Therefore, there is the possibility that prolonged exposure to silica contained in the outer membrane of breast implants may also induce ANCA-associated diseases. However, in one study, no typical symptoms were observed in ANCA-positive patients exposed to silica in their work environment. It was concluded that exposure to silica alone is insufficient to induce vasculitis. The presence of a family history and differences in disease prevalence between populations suggest some role of genetic predisposition in the pathogenesis of this entity [13].
Recently, an association between HLADRB1 0901 with microscopic polyangiitis (MPA) and ANCA-MPO-positive vasculitis in Japanese patients was reported. Unfortunately, a genotypic study of these patients was not conducted [14].
Bacterial infections have also been postulated as one of the triggering factors for diseases associated with ANCA. The role of Staphylococcus aureus in the production of ANCA was demonstrated in one study in which the immunization of rats with S. aureus produced ANCA in the circulation and caused segmental pauci-immune glomerular sclerosis. Additionally, a case of MPA and ANCA-MPO-positive vasculitis subsequent to bacterial endocarditis caused by S. aureus has been reported [15].
This all suggests some combination of genetic susceptibility, bacterial infections, and exposure to silica as a cause of MPA. As far as we know, Iyoda et al. reported the first case of MPA after the implantation of a silicone breast prosthesis, but no causal relationship has been clarified. Therefore, it is considered necessary to study the presence of ANCA and the genetic background of patients with silicone implants to establish if there really is a causal relationship.
Sjögren’s Syndrome
In a study conducted at University Hospital in Rotterdam, the Netherlands, investigators prospectively evaluated the prevalence of the complex of symptoms related to silicone and ANA in patients with breast cancer and silicone implants 1 year after implantation. In this study, a total of 57 women undergoing mastectomy followed immediately by reconstruction with breast implants were reassessed 1 year after their surgery, and 67% of these women had Sicca symptoms, mainly xerostomia and joint stiffness. However, they did not present with enough parameters to satisfy criteria for a diagnosis of Sjögren’s syndrome [16].
Still’s Disease
Still’s disease is a rare systemic inflammatory disorder of unknown etiology, characterized by fever, skin rashes, polyarthralgia, polyarthritis, hepatosplenomegaly, lymphadenopathy, leukocytosis, and elevated liver enzymes associated with high serum levels of ferritin.
The 5-year incidence of the disease has been estimated as 0.16 per 100,000 inhabitants. The clinical course can be classified into three main patterns: self-limiting or monophasic, intermittent or systemic polycyclic, and chronic articular. Several studies have analyzed the possible relationship between silicone breast implants and the subsequent development of several pathological conditions (adjuvant diseases), including Still’s disease [17].
Adjuvant, immuno-active factors like infectious agents, silicone, aluminum salts, and others are associated with the immunological substrate of this entity.
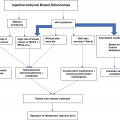
In recent years, four conditions – siliconosis, Gulf War syndrome, macrophagic myofasciitis, and post-vaccination phenomena – have been linked to previous exposure to an adjuvant. These four entities share a similar complex of signs and symptoms, which also constitute a common denominator. Therefore, Shoenfeld has suggested lumping these four conditions into a common syndrome, called autoimmune syndrome induced by adjuvants (ASIA).
Autoimmune Syndrome Induced by Adjuvants (ASIA)
Recently, this interesting syndrome was described by Shoenfeld in an article published in the Journal of Autoimmunity as an autoimmune, inflammatory syndrome induced by adjuvants. It incorporates several conditions that are not fully characterized as autoimmune diseases (like SLE, rheumatoid arthritis, or scleroderma), but which are induced by chronic stimulation of the immune system by substances that can act as adjuvants; among these adjuvants are bacterial antigens, hormones, aluminum, silicone, and several other molecules [18] (see Chaps. 8, 9 and 10).
This new syndrome developed after the publication of several studies on Gulf War syndrome, in which soldiers were described as suffering from atypical rheumatological symptoms — like arthralgia, myalgia, lymphadenopathy, malar exanthema, and chronic fatigue. This raised the question of whether vaccines administered to the soldiers had induced the symptoms. Recall that vaccines are immuno-active substances that contain viral or synthetic particles emulsified in adjuvants (such as aluminum), all intended to induce the immune reaction.
Macrophage myofasciitis is a condition that manifests as diffuse myalgias and chronic fatigue, both described in some cases of so-called Gulf War syndrome, which some consider to have been the result of the adjuvant effect of multiple vaccinations over the short period of time this war lasted. Those adjuvants were primarily aluminum hydroxide and squalene. Antibodies to squalene were detected in almost all patients with this syndrome [19].
There have also been papers describing autoimmune reactions post-vaccination with other types of vaccine, for example, vaccines for influenza A, human papilloma virus, hepatitis B, and tetanus; but these all are isolated cases. Post-vaccination autoimmunity is possible and should be taken into consideration when relevant symptoms appear, which is why the syndrome called ASIA is of clinical and research importance, at least as a means to place all the aforementioned disorders under a common umbrella. This being said, autoimmunity triggered by a vaccination is considered very rare.
With regard to silicone implants, it has been claimed that silicone is a totally inert substance, that it does not escape from its original capsule or travel through the body, that it does not induce granulomas, etc. But these claims are clearly incorrect, as there have been several recently reported cases of ruptured silicone implants. Moreover, even when silicone-containing implants do not rupture, silicone nanoparticles can be found in numerous different parts of the body, where they can cause localized or even widespread inflammatory reactions that induce the production of autoantibodies and the development of so-called adjuvant disease.
Similarly, autoimmune diseases that can be induced by infections are not as common as the infections themselves. This is due to some specific interaction between the infectious agent and the host’s genetic components and is linked to the incidence of autoimmune diseases.
With respect to ASIA, the prevalence is higher in those who carry the HLA-DRB1 gene. It should be noted that this is the same human leukocyte antigen (HLA) that was present in those who developed autoimmune disease after the administration of vaccines. Women with silicone implants and autoimmune diseases have been shown to exhibit differences in their HLA haplotypes relative to asymptomatic women without implants. The HLA DR and HLA DQ positive haplotypes have a very marked presence in women with silicone implants and systemic symptoms.
In a recent study, it was shown that, in susceptible individuals, a disturbance in the modulation of key cytokines may be responsible for the perpetuation of the inflammatory reaction, which locally causes capsular contracture and systemically may trigger autoimmune disease. In part, the mechanism by which adjuvants can cause these effects includes chronic stimulation of the immune system, which can then lead to the release of inflammatory cytokines, including interferon-γ, interferon-α, interleukin-1 (IL-1), IL-6, tumor necrosis factor (TNF)-α, and so on. So, in part, this syndrome can be induced by this cascade of cytokines being released in response to chronic stimulation.
This chronic stimulation may also involve opening of the blood-brain barrier and, therefore, the penetration of different substances into the brain. For example, one of the mechanisms that has been well defined is that aluminum, whether it comes from vaccines or exposure to other sources of aluminum, can cross the blood-brain barrier through macrophages and be deposited in brain tissue.
Chronic stimulation also induces the production of different autoantibodies, though these do not necessarily indicate a specific autoimmune disease. It could be that a combination of antibodies – like anti-DNA, which is more specific to SLE – is present, in conjunction with antimitochondrial antibodies that may indicate primary biliary cirrhosis. Over the years, undifferentiated connective tissue diseases can ultimately transform into a specific autoimmune disease. Therefore, it is also believed that most cases of undifferentiated connective tissue disease are actually part of ASIA.
Regarding the epidemiology of this syndrome, there is no knowledge about geographic distribution. We know that many autoimmune diseases are more frequent in populations that live far from the equator. It is believed that limited sun exposure and, therefore, reduced vitamin D production may also be associated with ASIA. We already know that vitamin D is associated with many autoimmune diseases. For example, in one study [20], more than 40 different autoimmune diseases were analyzed, and patients had significantly lower levels of vitamin D than the healthy population within the same geographical area; but these only were small numbers of cases. No large epidemiological studies have yet been able to analyze the geographic distribution of ASIA. However, it is believed that, over time, an association will be identified between ASIA and geographic location.
Suggested Diagnostic Criteria for ASIA
Exposure to an external stimulus (infection, vaccination, silicones, adjuvant) before the onset of clinical manifestations.
Presence of “typical” clinical manifestations:
Myalgia, myositis, or muscle weakness
Arthralgia or arthritis
Chronic fatigue, unrefreshing sleep, or sleep disturbances
Neurological manifestations (especially associated with demyelination)
Cognitive impairment or memory loss
Pyrexia and dry mouth

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree