Anorectal surgery
Hemorrhoidectomy/whitehead amputative hemorrhoidectomy
Stricture from coloanal or ileoanal anastomosis
Excision of low lying rectal tumors
Extensive debridement/fulguration of condyloma
Wide excision of Paget’s or Bowen’s disease
Trauma
Inflammatory bowel disease
Radiation
Infections
Sexually transmitted disease
Tuberculosis
Chronic laxative abuse
Neoplasia
Congenital abnormalities
Symptoms of anal stenosis are relatively consistent and are listed in Table41.2[1 4 12–14 17 18]. Diagnosis can be made by physical examination. An inability to pass an examining finger through the anus during digital rectal examination without discomfort or tearing the anoderm is diagnostic [3 4]. Patient discomfort may not allow adequate examination in the clinic or office setting, and examination under anesthesia may be required for diagnosis. Examination under anesthesia also can differentiate between functional narrowing of the anal canal, which will relax and open under anesthesia, and true anal stenosis caused by scarring, where the anal canal will remain stenosed [1].
Table 41.2
Symptoms of anal stenosis
Constipation |
Decrease in stool caliber |
Difficulty initiating evacuation |
Incomplete evacuation |
Tenesmus |
Diarrhea |
Bleeding |
Seepage and wetness (if associated with ectropion) |
The treatment of anal stenosis is dependent upon the severity and location of the stenosis. Milsom and Mazier [12] developed a simple classification system for anal stenosis (Table41.3). For cases of mild or moderate and low stenosis, nonoperative treatment with bulking agents and manual dilation, using digital examination in the office or with the aid of dilators used by the patient, may be all that is required [1 4 15]. However, there are few studies examining the efficacy of dilation in the treatment of this disease [1]. For more severe and higher stenosis, surgical intervention is required. Numerous surgical techniques have been described over the years and vary from simple stricture release with sphincterotomy to complex tissue flaps. This chapter focuses on various surgical techniques and their indications.
Table 41.3
Classification of anal stenosis
Classification by severity | Classification by location |
|---|---|
Mild: Exam can be completed with finger or medium Hill Ferguson retractor | Low: At least 0.5 cm distal to dentate line |
Moderate: Dilation need to examine with finger or medium Hill Ferguson retractor | Mid: 0.5 cm distal to 0.5 cm proximal to dentate line |
Severe: Unable to examine with little finger or small Hill Ferguson retractor unless forcefully dilated | High: At least 0.5 cm proximal to dentate line |
Preoperative Preparation and Postoperative Care
For most patients, minimal preoperative testing is required because many patients will not even tolerate an examination in the office. In the vast majority of patients, anal stenosis is related to prior surgery, and there is a valid concern regarding incontinence after surgery for anal canal resurfacing. This has led some authors to utilize preoperative anal manometry and endoanal ultrasound. Gonzalez et al. [17] attempted to perform manometry in 14 of 17 patients, 79 % of whom had normal resting and squeeze pressures. The remaining three patients could not tolerate testing because of pain. Endoanal ultrasound could only be performed in nine patients for the same reason. Seventy-eight percent had normal sonographic anatomy. Although preoperative testing was completed in at least half of the patients, the authors concluded that it is likely to be unnecessary in the majority of patients and should be reserved for those with previous sphincter defects or neurologic lesions.
Examination under anesthesia remains the most important and useful preoperative evaluation [1 13 17]. This allows the examiner to fully evaluate the severity and extent of the stenosis (anal verge, anal canal, or both) and to biopsy the stenotic area. Biopsy is typically not necessary in all cases of anal stenoses, but for those patients with a history of anorectal surgery for condylomata, Paget’s or Bowen’s disease, carcinoma, or Crohn’s disease, it is required to rule out recurrent pathology as the cause of the stenosis. Examination under anesthesia also will differentiate a functional stenosis from a true stricture caused by excessive scarring [1].
Once a diagnosis of moderate to severe stenosis has been made and nonoperative measures have failed to relieve the patient’s symptoms, surgery is indicated. The preoperative preparation is uniform. If the patient can tolerate it, a full bowel preparation or preparation with enemas the day before surgery is ideal. For many patients, this may not be tolerated and intraoperative rectal lavage may be required.
During the postoperative period, all patients undergoing surgery for anal canal stenosis traditionally have been kept on a constipating regimen for various periods of time to protect the wounds [4–6 9 11 18]. This is accomplished by maintaining patients on a liquid diet for 2–5 days with the addition of constipating agents. More recent studies have demonstrated that this regimen is not necessary [1 2 13 19 20], and patients can be placed on a high-fiber diet combined with stool softeners or bulk laxatives postoperatively. Patients typically are discharged home approximately 2 days postoperatively, depending on the extent of the procedure and the adequacy of their pain control.
Operative Procedures
Many procedures are described for the treatment of anal stenosis, but no one procedure has been identified as the “ideal” procedure because much of the literature does not compare one technique with another. According to Angelchik et al. [21], the ideal procedure is “one with minimal morbidity, good patient acceptance, simplicity of technique, and satisfactory long term results.”
The procedures described below are all performed preferably with the patient in the prone jack-knife position, with the buttocks taped apart to allow for adequate exposure while leaving enough skin exposed for mobilization of the flaps. A Foley catheter is inserted and all patients receive appropriate perioperative antibiotics. Regional anesthesia may be used in the majority of cases, although general anesthesia may be preferable for longer surgeries. Injection of a local anesthetic containing a diluted epinephrine solution is used to minimize bleeding. The lines of incision should be marked carefully with a sterile marking pen to ensure adequacy of mobilization for flaps.
Advancement Flaps
Mucosal advancement flaps similar to or variants of Martin’s technique, which originally was reported in 1944, have been described for the treatment of anal stenosis [3 14 18 22 23]. This technique involves excision of the scarred tissue, an internal sphincterotomy, and advancement of the rectal mucosa distally, which is then sutured to the dentate line.
Originally, these flaps were performed in the posterior quadrant [22 23], employing the technique of Khubchandani [3], which utilizes a lateral advancement flap. An initial incision is made laterally and perpendicular to the dentate line through the scar, extending to the anal verge. The scar then is excised and a distal internal sphincterotomy is performed. A mucosal flap 2–5 cm in length is raised, creating a transverse wound. The flap then is sutured transversely to the intersphincteric groove. Care should be taken to avoid suturing distal to this point because this may result in a troublesome mucosal ectropion. A small open wound in the perianal skin is left to heal by secondary intention. Utilizing this procedure, Khubchandani [3] obtained good outcomes in 82 % of patients. In another series, 90 % of patients had a good outcome, with reoperation required in only 10 % of cases [15]. The benefits of the mucosal advancement flap are that there is minimal morbidity (3 % in one study [15]) and, as opposed to skin flaps, the perianal wound is quite small. The use of a lateral rather than a posterior flap avoids the risk of keyhole deformity. The flap can be performed bilaterally for severe stenosis or contralaterally if the initial unilateral procedure fails [17]. The majority of patients may be discharged on the day of surgery [17].
The disadvantage of this procedure is that it can be technically challenging and can lead to an ectropion if the suture line is reconstructed too far distally [24]. This procedure may not be the procedure of choice for patients with severe distal stenosis because they seem to have a higher rate of restenosis – up to 20 % in one series [15]. Mucosal advancement anoplasty is ideal for mid- to upper anal stenosis where adjacent skin advancement flaps are preferred for the severe type of distal stenosis [16].
Y-V Anoplasty
The Y-V advancement flap is a pedicled skin flap originally described by Penn in 1944. The technique described by Gingold and Arvanitis [25] utilizes a Y-shaped incision, which is then sutured as a V. The most medial portion of the incision is the bottom of the Y, and the most lateral portion is the bifurcation of the Y (Fig.41.1). With this procedure it is imperative to measure the incisions carefully because the base of the flap (the most lateral portion of the incision or the top of the Y extending anterior to posterior) must be longer than the length of the flap (the length of the incision from medial to lateral, or the entire length of the Y) to ensure adequate mobility and to maintain the blood supply to the flap. Care must be taken to mobilize a full-thickness flap to avoid flap ischemia. The incision is initially made as a radial incision perpendicular to the stenosis (see Fig.41.1b). At this point an internal sphincterotomy may be performed [11]. The full-thickness flap then is raised along the upper limbs of the Y laterally and the resultant V-shaped flap is sutured to the wound edges, leaving no open wounds (see Fig.41.1c). Depending on the severity of the stenosis, this procedure may be performed unilaterally or bilaterally.
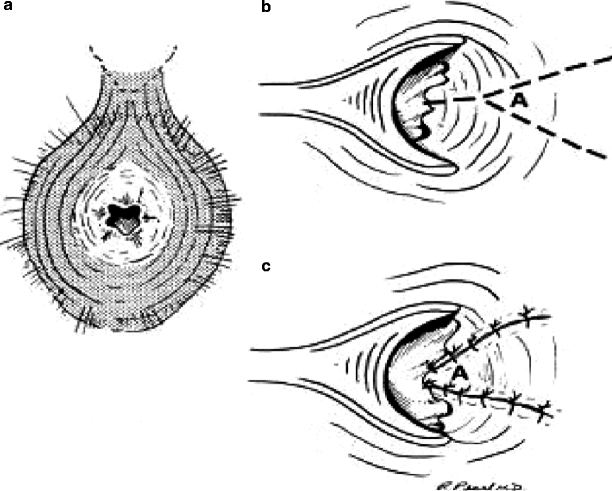

Fig. 41.1
Y-V anoplasty. (a) Anal canal with stenosis. (b) Line of incision for Y-V anoplasty. Note that the base of the incision from superior to inferior (i.e., the distance between the arms of the Y) should be equal to or greater than the length of the Y. (c) Completed Y-V anoplasty with all wounds closed
Success rates utilizing this technique range from 90 to 100 % [11 25]. In one series of 29 patients, early flap complications in three patients led to long-term unsatisfactory results [11]. The benefit of the Y-V anoplasty over other techniques is that it is a relatively simple technique and results in no open wounds. An obvious drawback to this procedure is that, because the flap is tethered at the lateral end, there may be tension on the medial aspect of the flap, resulting in ischemia and flap retraction. In addition, the blood supply is not uniform throughout the random pattern flap, which may cause ischemia and necrosis in the tip of the flap in the anal canal [26]. These complications may result in restenosis. In a study by Maria and associates [11], the only patients with poor long-term outcomes had either early suture dehiscence or ischemic contracture of the flap. Angelchik et al. [21] found that Y-V anoplasty had more complications than other techniques because of contracture, infection, or necrosis of the tip of the flap, thus limiting the utility of this flap for mid- and high stenosis. Its use is, therefore, best suited for low stenoses below the dentate line [1 20].
Diamond Flap
The operative technique of the diamond flap was first described by Caplin and Kodner [5] in 1986. In contrast to the previously described Y-V flap, dissection of the diamond flap is circumferential around the incision. There is no tethering to the adjacent skin, which allows the flap to be moved further into the anal canal for treatment of a higher stenosis. This technique and others similar to it have been termedisland flapsfor that reason [4]. Initially, the scar is released through a laterally based radial incision. If there is stenotic sphincter muscle, this incision can be deepened as an internal sphincterotomy. The resulting defect is approximately shaped as a diamond. At this point, the diamond-shaped flap is carefully drawn with a marking pen; the leading half of the flap (closest to the anus) should be the same size as the defect created with the initial incision (Fig.41.2a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








