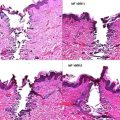
Skin is a complex organ covering the entire surface of the body. Aged skin is characterized by appearance of wrinkles, laxity, and pigmentary irregularities. These changes occur under the influence of intrinsic and extrinsic factors, with sun exposure being the most deleterious to the skin. Skin changes associated with aging are the focus of many surgical and nonsurgical procedures aimed to improve the appearance of skin. Knowledge of skin histology and physiology will deepen the understanding of cutaneous changes associated with aging and will promote optimal cosmetic and functional patient outcomes.
Skin is a complex organ covering the entire surface of the body. It provides a protective physical barrier between the body and the environment, preventing losses of water and electrolytes, reducing penetration by chemicals, and protecting against pathogenic microorganisms. The skin is important in regulation of body temperature and provides immunologic surveillance. It contains sensory and autonomic nerves and sensory receptors, which detect incoming stimuli of touch, vibration, pressure, temperature, pain, and itching.
Skin is an important component of outward beauty and is the focus of various cutaneous surgical and nonsurgical procedures. Skin changes associated with chronologic aging or photoaging, such as wrinkling, laxity, and changes in pigmentation, prompt patients to seek cosmetic procedures to improve the appearance of their skin. This article reviews skin histology and physiology as well as changes associated with cutaneous aging.
Normal skin architecture
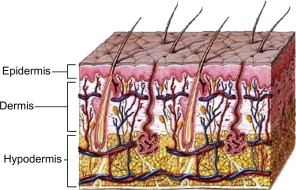
Skin is organized into 3 layers: the epidermis, the dermis, and the hypodermis ( Fig. 1 ). The fine structure of the skin shows considerable regional variations in epidermal and dermal thickness, distribution of epidermal appendages, and melanocyte content. Skin is glabrous or non–hair-bearing on the palms and soles, whereas hair-bearing skin covers the rest of the body.
Embryologically, the epidermis and its appendages develop from the surface ectoderm, and the dermis and hypodermis arise from mesoderm. During the fourth week of embryonic development, a single layer of ectoderm surrounds the embryo. This simple epithelium overlies a loosely organized layer of undifferentiated mesoderm known as mesenchyme. At about 6 weeks, the ectoderm and the underlying mesoderm begin to proliferate and differentiate. Hair follicles, nails, and glands begin to develop in the third month. By the end of the third month, regular bundles of collagen appear in the dermis. The embryonic connective tissue below the dermis develops into the subcutaneous layer of loose connective tissue characterized by fat islands.
The Epidermis
The normal epidermis is a stratified squamous epithelium undergoing continuous renewal. The major cell in the epidermis is the ectodermally derived keratinocyte, making up approximately 95% of the epidermal cells. As the keratinocyte progressively moves from its attachment to the basement membrane to the skin surface, it forms several morphologically distinct epidermal layers: stratum basale or stratum germinativum, stratum spinosum, stratum granulosum, and stratum corneum ( Fig. 2 ). On the palms and soles, an additional layer, stratum lucidum, can be identified between the stratum corneum and stratum granulosum. Other cell types found in the epidermis include melanocytes, Langerhans cells, and Merkel cells.
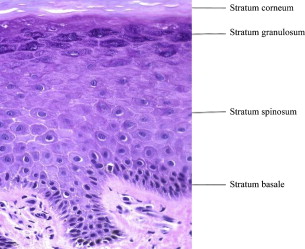
The basal layer is composed of a single layer of cuboidal cells, which rest on the basement membrane. Melanocytes can be seen between the basal cells of the epidermis. The basal cells divide, giving rise to the next layer: the prickle cell layer or stratum spinosum. This layer is usually 3 to 4 cells thick and is composed of polygonal cells with preformed keratin. The desmosomal attachments between the cells appear as small spines, giving rise to the name stratum spinosum. The stratum spinosum is succeeded by stratum granulosum, which is usually 1 to 4 cells thick and derives its name from cytoplasmic keratohyalin granules. The outermost layer of the epidermis is stratum corneum composed of flattened keratinocytes that have lost their nuclei and cytoplasmic organelles. These keratinocytes are shed in the process of epidermal turnover.
Melanocytes are derived from neural crest cells and migrate into the epidermis where they produce melanin. They are distributed among the basal keratinocytes with the ratio of 1 melanocyte to 4 to 10 basal cells. This ratio varies with anatomic location, with maximal density of melanocytes on genital skin. Melanin is produced from tyrosine by the enzymatic activity of tyrosine kinase and is stored in melanosomes. Melanosomes are transported along the dendritic processes of melanocytes to adjacent keratinocytes, where they form an umbrella-like cap over the nucleus, protecting it from the injurious effects of UV light. The ethnic variations in pigmentation are attributable to the different activity of melanocytes, not the difference in number of melanocytes. With age, melanocyte density decreases by 6% to 8% per decade, with higher density of melanocytes in sun-exposed skin than in non-exposed skin at all ages. This explains the generalized increase in pigmentation and simultaneous decrease in melanocyte density that often accompany aging.
Langerhans cells are antigen-presenting cells derived from bone marrow. They make up 3% to 6% of all cells in the epidermis and are found mainly within the spinous layer. The dendritic processes of Langerhans cells uptake antigens deposited on the skin, process them, and present them to T lymphocytes for activation of the immune response. The number of Langerhans cells increases during allergic reactions, such as contact hypersensitivity. With aging and chronic sun exposure, the number of Langerhans cells decreases, which may play a permissive role in the development of cutaneous carcinoma in individuals who are elderly with sun-damaged skin.
Merkel cells are found in the basal layer of the epidermis and in the epithelial sheath of hair follicles. Merkel cells migrate from neural crest to the skin and have similar chemical and structural properties to an amine precursor uptake decarboxylation (APUD) cell. Merkel cells are associated with sensory nerve endings in the skin and may function as mechanoreceptors.
Epidermal appendages
Epidermal appendages are specialized epithelial structures located mainly in the dermis and hypodermis, but connected to the epidermis. They include pilosebaceous follicles, sweat glands, and apocrine glands. They play an important role in the epithelialization phase of wound healing.
Pilosebaceous unit
The pilosebaceous unit consists of hair, hair follicle, sebaceous gland, and arrector pili muscle ( Fig. 3 ). It is distributed throughout the integument, absent only in palms and soles and portions of the genitalia.
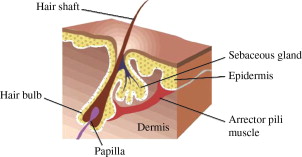
The hair follicle is composed of several segments: infundibulum, isthmus, lower follicle, and hair bulb. The topmost portion is the infundibulum, extending down from the skin to the opening of the sebaceous duct into the hair follicle. The isthmus extends from the infundibulum to the bulge, the site of insertion of the arrector pili muscle into the follicle. The bulge contains stem cells of the hair follicle. Contraction of the arrector pili muscle causes the hair to be pulled from an oblique to a more vertical orientation in response to stimuli from the sympathetic nervous system. Clinically, contraction of the arrector pili muscle results in slight elevations of the skin or goose bumps. The lower follicle extends down to the top of the hair bulb and the hair bulb is the terminal portion of the hair follicle, which envelops the follicular papilla.
The papilla is a dermal structure containing a richly vascularized and innervated connective tissue and fibroblasts, important for hair growth. The hair bulb contains the matrix cells that give rise to the hair and melanocytes responsible for hair pigmentation. Permanent hair removal requires damage to follicular stem cells in the bulge region of the hair follicle.
Hair growth exhibits a cyclical pattern. Anagen is the growth phase in which cells of the hair matrix are dividing rapidly. Catagen is the involutional phase in which cell division ceases, hair matrix regresses, papilla retracts to the level of the insertion of the arrector pili muscle, and capillary nourishment diminishes. During the resting phase, known as telogen, the follicle detaches from the papilla and contracts, eventually falling out. At any given time in one anatomical location, follicles are present in different phases of the cycle. Hairs in the anagen phase are susceptible to destruction by laser; therefore, repeated hair removal treatments are necessary to address new follicles coming into the anagen phase.
With aging, the rate of hair growth declines and hair diminishes in thickness. Gray hair appears as the result of decreased melanin production within the hair matrix.
Sebaceous glands empty into the hair follicle. In some areas, sebaceous glands are not associated with a hair follicle and open directly onto the skin’s surface. These areas include the eyelids, areolae of the nipples, and vermilion border of the lips.
Sebaceous glands are present in most areas of the body with the exception of palms and soles, with highest density of sebaceous glands found on the face. They consist of an outer layer of basal cells and several layers of lipid-laden sebocytes. Sebocytes disintegrate toward the center of the gland, thus forming sebum, which empties into the hair follicle via a short duct. Sebum provides emollients for the hair and skin. The secretions of the sebaceous glands are under the influence of androgen hormones. The gland activity increases shortly after birth and subsides in the first few postnatal months. At puberty, there is a new rise in sebaceous gland activity associated with increased androgen output. Increased sebum production is a major factor in the pathophysiology of acne vulgaris. Sebum secretion declines with age by about 23% per decade in men and 32% per decade in women. The size of the sebaceous glands increases with age, despite decrease in sebum production. This can result in large pores, as seen with age.
Sweat glands
The eccrine glands are the main sweat glands in humans and play a vital role in the process of thermoregulation. They are found almost everywhere on the skin except the vermillion border of the lips and nail beds and have maximum density over palms, soles, axillae, and forehead. With aging, the number and function of eccrine sweat glands decrease.
Each eccrine gland is composed of 3 parts: the secretory coiled gland, the dermal duct, and the intraepidermal spiral duct known as acrosyringium. The secretory gland is situated in the deep dermis or at the dermal-hypodermal junction. It is composed of secretory clear and dark cells surrounded by contractile myoepithelial cells. Clear cells produce sweat in response to cholinergic stimulation. Their secretions are discharged into the lumen of the gland through intercellular canaliculi. The excretory duct ascends through the dermis in a vertical fashion and spirals through the epidermis, opening directly onto the skin’s surface.
Apocrine glands form as outgrowths of the pilosebaceous units. Unlike eccrine glands, they are present primarily in the axillae and in the anogenital region. Apocrine glands are also found in other areas of the body, such as the external auditory canal, eyelid, and breast. The apocrine gland consists of a coiled gland located in the subcutaneous fat, and a straight duct that ascends through the dermis and empties into the infundibulum of the hair follicle. The coiled gland consists of one layer of secretory cells around a large lumen, surrounded by myoepithelial cells. Apocrine glands produce secretions by decapitation, a process where the apical portion of the secretory cell’s cytoplasm pinches off and enters the lumen of the gland. Apocrine sweat is odorless initially and develops an odor when it comes in contact with bacterial flora on the surface of the skin.
The dermal-epidermal junction
The dermal-epidermal junction is a basement membrane synthesized by basal keratinocytes and dermal fibroblasts. It functions as a mechanical support for adhesion of dermis to the epidermis and as a barrier to chemicals and cells. The structure of the dermal-epidermal junction is complex, consisting of 4 distinct layers seen on electron microscopy. The top layer contains the cell membrane of basal keratinocytes and their hemidesmosomes. Cytoskeletal filaments course through the basal cells and insert into the hemidesmosomes. The next layer is lamina lucida, which is traversed by anchoring filaments, and rests on top of lamina densa. The sub-basal lamina layer is a filamentous zone mainly made up of anchoring fibrils, which serve to anchor the epidermis and dermal-epidermal junction to the dermis.
The Dermis
The dermis is divided into 2 distinct regions: the upper papillary dermis and the lower reticular dermis. The papillary dermis is located just below the dermal-epidermal junction and forms conic upward projections known as dermal papillae. These projections interdigitate with epidermal rete ridges, increasing the area of dermal-epidermal interface and allowing for better adhesion between dermis and epidermis. The papillary dermis is composed of loosely arranged bundles of collagen, elastic fibers, fibrocytes, blood vessels, and nerve endings. The reticular dermis contains compact collagen fibers, thicker elastic fibers, deep part of epidermal appendages, and vascular and nerve networks.
Collagen is the major constituent of the dermis and is made of collagen fibers synthesized by fibroblasts. More than 90% of dermal fibers are made of interstitial collagen, mainly types I and III, providing tensile strength and mechanical resistance to the skin.
Elastic fibers are responsible for returning skin to its normal configuration after deformation and extend from lamina densa of the epidermal-dermal junction into the reticular dermis. The elastic fibers are thin in the papillary dermis but become thicker and more horizontally oriented in the reticular dermis. Reticulin fibers are also present in the dermis and consist of thin collagen fibers and fibronectin.
Dermal fibers and cells are embedded in the matrix of macromolecules known as ground substance. It consists of glycoproteins and proteoglycans and is abundant in the papillary dermis.
Cellular components of the dermis include fibroblasts, dermal dendrocytes, and mast cells. Fibroblasts are the main cells in the dermis synthesizing dermal fibers and the ground substance. They are abundant in the papillary dermis, but fewer in number in the reticular dermis. Fibrocytes are quiescent fibroblasts devoid of metabolic activity. Fibroclasts are fibroblasts with phagocytic activity toward collagen. Myofibroblasts are derived from fibroblasts and are involved in wound healing. Mast cells are derived from bone marrow and are distributed around blood vessels and adnexal structures of the papillary dermis. Dermal dendrocytes represent a heterogeneous population of mesenchymal dendritic cells and may act as phagocytes and antigen-presenting cells.
Vasculature
The skin has a rich vasculature consisting of 2 horizonally arranged networks: a superficial and a deep subdermal plexus. These networks originate from the perforator arteries of the underlying muscles and communicate via small vessels traversing the dermis vertically ( Fig. 4 ). The subdermal plexus lies near the dermal-hypodermal junction and supplies hair follicles and sweat glands. The superficial plexus is derived from terminal arterioles and lies at the interface between the papillary and reticular dermis. The superficial plexus gives rise to a vascular loop that ascends to every dermal papilla as a precapillary arteriole, forms a turn of arterial and venous capillaries, and descends into the papillary dermis as a postcapillary venule. The venules form the superficial venular plexus, which connects with veins in subcutaneous fat. In some areas, arterioles and venules communicate directly via arteriovenous shunts bypassing the capillary circulation. These shunts are controlled by glomus cells innervated by adrenergic fibers.