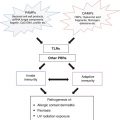
Phenotypes
Major immune cells
Major cytokines or cytotoxic mediators
MPE
CD4+ > CD8+ T cells
IFN-γ, TNF-α, IL-4, IL-5, perforin/granzyme B
DRESS
CD4+ > CD8+ T cells, eosinophils
IFN-γ, TNF-α, IL-4, IL-5, TARC/CCL17
SJS/TEN
CD8+ T cells, NK cells
IFN-γ, TNF-α, Fas-FasL, perforin/granzyme B, granulysin
AGEP
Neutrophils
IL-8
Skin manifestations of DRESS may vary from MPE-like to exfoliative dermatitis and are characterized by a heavy infiltration of CD4+ and CD8+ T cells, monocyte/macrophages and eosinophils [21]. MPE and DRESS share many pathological features, but DRESS exhibits more severe dyskeratosis (keratinocyte death in epidermis) and a greater extent of systemic involvement and eosinophilia [26].
Immunohistology of skin lesions in AGEP reveals intraepidermal pustules with infiltration of neutrophils surrounded by IL-8 producing T cells [22].
Despite very diverse clinical presentations, constant features of delayed-type drug hypersensitivity are the presence of high numbers of drug-specific CD8+ cytotoxic T cells and low numbers of innate NK lymphocytes [20, 27, 28].
CD8+ T cells of cutaneous ADRs have classic cytotoxic functions: lysis of autologous lymphocytes or keratinocytes in an MHC class I–restricted and drug-dependent manner [28].
Cytotoxic Immune Cells in SJS/TEN
Drug-induced SJS and TEN are severe cutaneous ADRs in which cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are activated, and subsequently carry out the cellular immune reactions directed at keratinocytes in a major histocompatibility class (MHC) I-restricted manner. Upon activation of these immunocytes, various cytotoxic signals, including granulysin, perforin/granzyme B, Fas/Fas ligand, and cytokines/chemokines, are relayed to the skin lesions to mediate the disseminated keratinocyte death [23–25]. It is noteworthy that the number of granulysin-positive cells in fixed drug eruptions was found to be similar to that observed in SJS/TEN [27].
Non-Immune-Mediated Hypersensitivity
Non-immune-mediated hypersensitivity is commonly referred to as pseudoallergic reactions because they do not involve a specific immune mechanism – neither IgE-mediated (Type I) nor delayed (Type IV) hypersensitivity. Clinical manifestations, which range from milder erythematous to urticarial reactions to severe lethal anaphylaxis, may be indistinguishable from immune system-mediated hypersensitivity reactions. Common non-immune-mediated hypersensitivity can be caused by contrast media, vancomycin, non-steroidal anti-inflammatory drugs (NSAIDs), opiates, plasma expanders, and drugs used in general anesthesia [29].
NSAIDs-induced pseudoallergic reactions have been attributed to cyclooxygenase-1 inhibition and overproduction of leukotrienes, and may require higher drug doses than are needed for true IgE-mediated reactions [30]. Mast cell degranulation is involved in some of these pseudoallergic reactions.
The Role of Cytokines or Inflammatory Mediators
Drug-specific T cells mediate skin inflammation in variable clinical presentations of delayed-type drug hypersensitivity through the release and induction of different cytokines and chemokines (Table 25.1) [31]. The heterogeneous cytokines include Th1 cytokines (interferon-γ) and Th2 cytokines (IL-4, IL-5) [22]. Increased expression of IL-5, which is a key cytokine for activation of eosinophils, is commonly seen in delayed-type drug hypersensitivity [32]. The activation of eosinophils can be further enhanced by the chemokines eotaxin and RANTES [33]. Thymus and activation-regulated chemokine (TARC/CCL17) has been reported to be a DRESS specific cytokine [34]. In addition to Th1 and Th2 cytokines, a recent study demonstrated the involvement of IL-17A-producing Th17 in DRESS and SJS/TEN [35]. Elevated expression of the neutrophil-attracting IL-8 has been known to be the key cytokine involved in AGEP.
There are several cytokines involved in SJS/TEN. Numerous studies have shown tumor necrosis factor alpha (TNF-α) strongly expressed in SJS/TEN lesions and correlated with disease severity [24, 36, 37]. TNF-α is a potent cytokine that induces cell apoptosis, cell activation, differentiation, and inflammatory processes [38, 39]. Interferon gamma (IFN-γ) is a common cytokine involved in delayed-type drug hypersensitivity, including SJS/TEN. IFN-γ was intensely expressed in the superficial dermis and epidermis of SJS/TEN lesions [36, 37]. IFN-γ is also known to promote antigen presentation and thus stimulate the cell-mediated immunity by upregulation of MHC molecules [40–42]. In addition to TNF-α and IFN-γ, several cytokines and chemokine receptors that are responsible for trafficking, proliferation, and activation of T-cells and other immune cells have been found elevated in the skin lesions, blister fluids, blister cells, PBMCs, or plasma of SJS/TEN patients. These cytokines/chemokines include IL-2, IL-5, IL-6, IL-10, IL-12, IL-13, IL-15, IL-18, CCR3, CXCR3, CXCR4, and CCR10 [24, 36, 37, 43–45].
Immune Mediators for Cell-Mediated Cytotoxicity in SJS/TEN
The central hypothesis proposed to explain the severe mucocutaneous lesions of SJS/TEN is the CD8+ cytotoxic T cell and natural killer (NK) cell-mediated cytotoxic immune reactions. Three major cytotoxic signals from cytotoxic cells are reported to be involved in the extensive skin necrosis of SJS/TEN, including the Fas–FasL interaction, perforin/granzyme B, and granulysin, which can induce keratinocyte apoptosis [23, 28, 46].
Granulysin is not only a cytotoxic protein; it is also a chemoattractant and proinflammatory activator that can promote monocyte expression of CCL20 [47], and is capable of promoting antigen-presenting (dendritic) cells and leukocyte recruitment, and activating specific immune responses, such as IL-1b,IL-6, IL-10, TNF-a [48].
Genetic Predisposition
Genetic Factors in Delayed-Type Drug Hypersensitivity
Reports on the familial occurrence of severe drug hypersensitivity and cases occurring in identical twins suggest genetic links [49–52]. The HLA genes show strong association with drug hypersensitivity. Examples of strong associations of HLA alleles with specific drug-induced hypersensitivity reactions include abacavir, nevirapine, carbamazepine, and allopurinol (Table 25.2).
Table 25.2
Recently reported HLA associations with drug hypersensitivity reactions
Drug | HLA association | Hypersensitivity reactions | Reference |
|---|---|---|---|
Carbamazepine | B*1502 | SJS/TEN | Chung et al. [53] |
Allopurinol | B*5801 | SJS/TEN/DRESS | Hung et al. [54] |
Abacavir | B*5701 | MPE/DRESS | Mallal et al. [55] |
Flucloxacillin | B*5701 | Hepatotoxicity | Daly et al. [56] |
Lumiracoxib | DRB1*1501, DQB1*0602, DRB5*0101, DQA1*0102 | Hepatotoxicity | Singer et al. [57] |
Dapsone | B*1301 | MPE/DRESS | Zhang et al. [58] |
Nevirapine | DRB1*0101 | MPE/DRESS | Martin et al. [59] |
Methazolamide | B*5901 | SJS/TEN | Kim et al. [60] |
The view that HLA alleles are the main genetic determinants of SJS/TEN was first proposed by Roujeau et al. [61], who reported the weak associations of HLA-A29, B12, and DR7 in sulfonamide-related TEN, and HLA-A2, B12 in oxicam-related TEN in Europeans [61]. Following the immunological hypothesis, the most striking evidence of genetic susceptibility to SJS/TEN was provided by the findings that HLA-B*15:02 is strongly associated with carbamazepine-induced SJS/TEN [53], HLA-B*58:01 with allopurinol-induced SJS/TEN or DRESS [54], and HLA-B*5701 with abacavir hypersensitivity [62].
The HLA association to specific drug-induced hypersensitivity can be ethnic and phenotype-specific. The strength of HLA associations with specific drug-induced hypersensitivity in different populations has been found related to the prevalence of the susceptibility allele in the ethnic population. The association of HLA-B*15:02 with carbamazepine-induced SJS/TEN was replicated in other Asian countries, including Thailand, Hong Kong, Malaysia, China, Vietnam, Cambodia, Reunion, Philippines and Indian ethnicities, which carry high HLA-B*15:02 allele frequency, but not in Europeans, which carry low HLA-B*15:02 allele frequency (<1 %) [63]. In contrast, the strong association of HLA-B*58:01 with allopurinol-induced SJS/TEN is more universal, being found in Han Chinese in China, Thai populations, Korean, Japanese, and European populations; HLA-B*58:01 is the allele common to all these populations [64]. The phenotype-specific characteristics are exemplified by carbamazepine hypersensitivity. While HLA-B*15:02 is strongly associated with carbamazepine-induced SJS/TEN, it is not associated with carbamazepine-induced DRESS; in an international study, HLA-A*31:01was strongly associated with carbamazepine-induced DRESS, but not with carbamazepine-induced SJS/TEN [65].
Phenytoin – an aromatic antiepileptic drug structurally related to carbamazepine – also frequently causes SJS/TEN and DRESS [66, 67]. HLA-B*15:02 has been associated with phenytoin-related SJS/TEN in Asians, although the association is much weaker than that found for carbamazepine-related SJS/TEN [68]. A recent genome-wide association study by Chung WH et al. turned up cytochrome (CYP) 2Cvariants, including CYP2C9*3, that showed a strong association with phenytoin-related SCAR. The significant association between CYP2C9*3 and phenytoin-related severe cutaneous ARDs was replicated in different Asian populations [69].
Genetic Factors in Immediate-Type Drug Hypersensitivity
Similar to delayed-type drug hypersensitivity, genetic predisposing factors have been reported in immediate-type drug hypersensitivity. β-lactam allergy was reported associated with gene variants of IL13, IL4, and IL4RA [70–73]. Several genetic predisposing factors, including gene polymorphisms in cysteinyl leukotriene receptor type 1 (CysLTR1) and leukotriene C4 synthase (LTC4S) [74] and high-affinity IgE receptor (FcepsilonR1) [75], were associated with aspirin.
Classification
Cutaneous ADRs may be classified in terms of their presumed mechanism, severity of the reaction, histological findings, and cutaneous morphological manifestations.
Mechanism of ADRs
The modern pharmacological classification of ADRs differentiates two basic types of reactions; type A, predictable reactions, and type B, unpredictable or idiosyncratic reactions. Type A reactions (‘augmented’) are dose-dependent, common and predictable based on the pharmacology of the drug; about 80 % of all ADRs are type A. Type B reactions (‘bizarre’) do not occur at any dose in most patients, but may be dose dependent in susceptible individuals. They are uncommon, affecting a small number of patients based on an individual predisposition that depends on both genetic and environmental factors [76, 77].
The pathogenesis of Type A reaction was described in the sixteenth century by Paracelsus, the Swiss German Renaissance physician who founded the discipline of toxicology: “All things are poison, and nothing is without poison; only the dose permits something not to be poisonous” [78]. The pathogenesis of Type B reaction was designated in the first century BC didactic poem, De rerum natura (On the Nature of Things), by the Roman poet and philosopher Lucretius: “One man’s meat is another man’s poison” [79].
Type B reactions can be categorized into different subtypes according to Gell and Coombs’ classification system [80]. The effector phase of the allergic reaction is classified into four types: Type I mediated by drug-specific IgE antibodies, Types II and III mediated by drug specific IgG or IgM or IgA antibodies, and Type IV induced by drug-specifc T lymphocytes [81]. This classification system may be helpful in daily clinical practice as a guide to diagnostic and therapeutic decisions.
In addition to the basic classification of Type A and B reactions, further types of reactions were subsequently added; Type C- dose and time-related, ‘Chronic’; Type D- time-related. ‘Delayed’; Type E- withdrawal effects, ‘End of use’; and Type F- unexpected failure of therapy, ‘Failure’ [2].
Severity of Cutaneous ADRs: Skin only (Simple) Versus Skin and Systemic Involvement (Complex)
The diagnosis of a cutaneous ADR must be followed by differentiation between a simple reaction involving only the skin and a complex reaction that includes systemic involvement of organs in addition to the skin [82]. Systemic involvement should be explored even in a mild cutaneous eruption due to a drug since the severity of skin manifestation does not necessarily mirror the severity of the systemic involvement. Systemic involvement is evaluated by assessing the patient’s symptoms, including fever, facial edema, malaise, chills, dyspnea, cough, palpitations, nausea, vomiting, diarrhea, sore throat and arthralgia. Further investigation is based on the patient’s symptoms. Basic laboratory screen, conducted in cases of suspected systemic involvement, includes a full blood count, liver and renal function tests, and urine analysis [83].
Histological Classification of Cutaneous ADRs
Skin biopsy is an invaluable diagnostic modality in the assessment of drug eruptions. Histologically, drug eruptions can elicit a variety of inflammatory disease patterns in the skin and panniculus, and overlapping reaction patterns. Ackerman et al.’s basic patterns of inflammatory skin diseases [84] (Table 25.3) are a helpful guide. The most common pattern of drug eruptions is the perivascular type, while psoriasiform and granulomatous patterns are rarely reported [85]. Drug eruptions may also mimic specific skin diseases such as lupus, lichen planus or lymphoma [85]. A single drug may cause a wide range of reaction patterns and no reaction pattern is specific for a particular drug [88]. While the histological changes are not distinctive in many cases of drug eruption, a few important histopathological clues may aid in the diagnosis: (1) Overlapping histological patterns in one specimen (e.g., lichenoid and spongiotic). (2) Presence of eosinophils (although not mandatory); although eosinophils are an important tell-tale sign of a drug-induced reaction, they may also be conspicuous in skin rashes devoid of a drug association and sparse or absent in some drug exanthems. (3) Apoptotic keratinocytes. (4) Mismatch between clinical and histomorphological features [85, 86, 88].
Perivascular | Superficial perivascular | Mixed infiltrate | Spongiotic | Psoriasiform | Interface pattern |
|---|---|---|---|---|---|
Purpuric drug eruption | Urticarial drug eruption | Pityriasis rosea–like eruption Photosensitive drug eruptions: Phototoxic reaction Photoallergic reaction | Psoriasiform drug eruption | Vacuolar: EM SJS TEN FDE Morbilliform drug eruption Lupus erythematosus-like eruption Chemotherapy-induced interface dermatitis Lichenoid drug eruption | |
Nodular and diffuse | Lymphomatous | Neutrophilic | Granulomatous drug eruptions | ||
Pseudolymphomatous drug reaction | Drug-induced Sweet syndrome | Interstitial granulomatous drug reaction (IGDR) Drug-induced accelerated rheumatoid nodulosis Drug-induced granuloma annulare Drug-induced sarcoidosis | |||
Vesiculobullous | Drug-induced linear IgA bullous dermatosis | Drug-induced pemphigus | Drug-induced bullous pemphigoid | Drug-induced pseudoporphyria cutanea tarda | |
Pustular | AGEP | ||||
Vasculitis | Drug-induced vasculitic reaction | ||||
Folliculitis and perifolliculitis | Acneiform drug eruptions | Drug-induced eosinophilic pustular folliculitis (Ofuji’s disease) | |||
Fibrosing dermatitis | Sclerodermoid drug reaction | ||||
Panniculitis | Drug-induced panniculitis | ||||
In a study assessing the histological pattern of 104 cases of diagnosed drug eruption during a 5-year period in one institution [89], the majority of the cases (94 %) were morbilliform-type rashes. The most common histological pattern was superficial perivascular and interstitial with interface changes. Eosinophils were present in only 50 % of cases, and approximately half (53 %) of the cases exhibited epidermal-dermal interface changes [89].
In view of the large diversity of cutaneous drug reactions, it is helpful to approach them as clinicopathologic entities and to base the diagnosis on a combination of clinical, histological and disease course data [89]. Heightened awareness of the possible mimicry of other skin diseases and of the suspicious histopathological clues pointing to drug etiology are key elements to the appropriate histological diagnosis of drug reactions in the skin [85, 88, 89].
Morphological Classification of Cutaneous ADRs
A widely accepted approach to diagnosing the type of drug eruption is a simplified method based on the morphology of the primary lesions. The four main categories are maculopapular, urticarial, pustular and blistering [82]. The diagnosis of the drug eruption can be challenging since the same cutaneous morphology can be manifested in a simple reaction involving only the skin and in a complex reaction including systemic involvement in addition to the skin. Therefore, there are two major steps in diagnosing drug eruptions: determine the morphology and assess systemic involvement [90].
Maculopapular Eruptions – MPE (Synonyms: Morbilliform, Exanthematous)
Terminology
The term ‘maculopapular’ is descriptive. Morbilliform means measles-like, the rash of measles consisting of macules and papules that tends to confluence. The etymon of ‘exanthema’ is the Greek ‘exanthema’, which means ‘a breaking out’. Thus exanthema merely means ‘rash’, and ‘exanthematous rash’ literally means ‘rash-like rash’. Therefore, the terminology is redundant [89].
Skin Signs
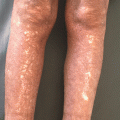
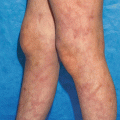
Polymorphous pink-to-red macules and or papules usually in a symmetric distribution that may coalesce to form plaques (Fig. 25.1) [91]. The eruption begins on the trunk and upper extremities and progressively becomes confluent. In addition, purpuric lesions may appear on the ankles and feet [90]. The drug eruption can also manifest in a scarlatiniform pattern of pinpoint-sized pink-red papules coalescing and giving the skin the texture of sandpaper [92].
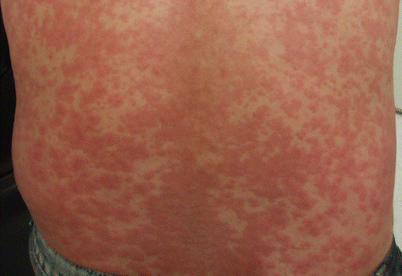

Fig. 25.1
Erythematous macules and papules coalescent into ill-defined plaques on the trunk – maculopapular morphology of cutaneous ADR
Maculopapular Eruptions – MPE – Simple (Skin Only)
Frequency
The most common drug-induced eruptions, occurring in 1–5 % of first-time users of most drugs [91].
Common Sites of Involvement
The eruption usually begins on the trunk and becomes generalized. Palms and soles are often involved; mucous membranes are usually spared [90].
Histology
Nonspecific changes consisting of mostly superficial but also deep perivascular and interstitial infiltrate of lymphocytes. Eosinophils and epidermal-dermal interface changes appear in approximately half the cases [89].
Differential Diagnosis
viral exanthems, scarlet fever, toxic shock syndrome, acute graft versus host disease (GVHD), Kawasaki disease, juvenile idiopathic arthritis [90].
Treatment
Identifying and discontinuing the causative drug are the most important steps in management. Symptomatic treatment with antipruritic agents and potent topical glucocorticoids may be helpful [91]. A decision can be made to continue the drug and offer symptomatic treatment if the drug is of paramount importance, but the risk: benefit ratio of this option has to be carefully weighed, and the evolution of the eruption must be meticulously monitored [90].
Prognosis
The eruption often fades within 7–14 days of discontinuation of the offending drug and scaling and desquamation may follow. Re-challenge may lead to reappearance of the reaction within a few days [90].
Offending Drugs
The most common classes of drugs implicated are penicillins, sulfonamides, cephalosporins, and antiepileptics [90].
Maculopapular eruptions – MPE – Complex (skin + systemic involvement): DRESS – See Severe Cutaneous Adverse Drug Reactions.
Urticarial Eruption
Terminology
The term ‘urticaria’, first introduced by William Cullen in the eighteenth century, is derived from urtica urens (common European stinging nettle). One of the earliest descriptions of urticaria comes from China, and is more than 2,000 years old. In the Huangdi Neijing, written around 200 BC, urticaria is referred to as Feng Yin Zheng (‘wind type concealed rash’). In ancient Latin medical literature, urticaria was called ‘uredo’ (urere means ‘to burn’), and in the old Persian medical texts, ‘essera’ (meaning ‘elevation’) [93].
Skin Signs
Urticaria is induced by superficial dermal swelling due to plasma leakage and vasodilation triggered by activation of mast cells. The skin manifestations of this process include erythematous and edematous papules and plaques (wheals) of various sizes that may coalesce to form large plaques [94]. Wheals may be characterized by pink or pale center and assume a figurate or polycyclic configuration. Linear lesions can be seen with dermatographism [92, 94].
Urticarial Eruption – Simple (Skin Only)
Frequency
Drug-induced urticarial eruptions are the second most common type of cutaneous drug eruption and account for approximately 5 % of all cutaneous drug eruptions [85].
Symptoms
A major clinical feature is pruritus, the lack of which should put the diagnosis in doubt. The lesions can also be painful if they occur on the soles, over joints, or in areas where the skin is tightly adhered to subcutaneous tissue [94]. A single lesion lasts less than 24 h and upon resolution leaves normal skin. However, new lesions may continue to arise for various periods of time. Acute urticaria is defined when a bout of hives lasts less than 6 weeks; when it lasts longer, it is defined as chronic urticaria [95].
Urticaria may be associated with angioedema [93]. Angioedema is defined as a deep, dermal, subcutaneous and/or mucous swelling that may involve the intestinal lining and the upper respiratory tract. Symptoms include slight heat, burning, pain and sensation of pressure or tightness. However, pruritus is minimal or absent. Swelling of gastrointestinal tract mucosa can induce abdominal pain, vomiting and diarrhea. Edema of the respiratory tract may induce various symptoms including life-threatening asphyxia. Drug-induced angioedema is associated with urticaria in approximately 50 % of cases. Some drugs may induce angioedema without urticaria [96].
Common Sites of Involvement
Lesions of urticaria can appear anywhere on the skin, including the palms, soles and scalp, but not on mucosal surfaces [94]. Angioedema most commonly occurs in the head, neck and hands, but can occur anywhere and frequently involves mucosal tissue. Swelling may be more prominent in areas of looser skin, such as the scrotum, labia, lips, and eyelids [94].
Histology
Urticarial drug reactions are characterised by dermal edema and a superficial and deep perivascular and interstitial dermatitis. The mixed inflammatory infiltrate comprises lymphocytes, histiocytes, mast cells, eosinophils and neutrophils. The presence of neutrophils and deep vascular plexus involvement may be a clue to the drug-induced nature of the urticaria [86].
Differential Diagnosis
The wheals with central red halo of urticaria may resemble the target lesions of erythema multiforme. Four clinical signs of urticaria can help distinguish it from erythema multiforme: (1) The central zone consists of normal skin, whereas in erythema multiforme, skin is dusty, bullous or crusted. (2) Each lesion is transient, lasting less than 24 h, whereas erythema multiforme lesions are ‘fixed’ for a few days. (3) New lesions appear daily and in erythema multiforme all lesions appear within the first 72 h. (4) There may be associated swelling of face, hands and feet and in erythema multiforme there is no edema [97]. Differential diagnosis of urticaria includes also bullous pemphigoid, urticarial vasculitis and serum sickness-like reaction (SSLR). Drug-induced urticaria needs to be differentiated from cases of urticaria induced by other etiologies, such as food, environmental allergens, insects, systemic illness, physical stimuli, genetic and idiopathic [94].
Urticaria and angioedema are the most common symptoms of anaphylaxis (88 % of cases), and are one of the clinical criteria of the National Institute of Allergy and Infectious Disease (NIAID) and the Food Allergy and Anaphylaxis Network (FAAN) for the diagnosis of anaphylaxis [98]. Therefore, all cases of sudden acute urticaria and angioedema should be evaluated for indications of the anaphylactic type of reaction: presence of respiratory compromise, decreased blood pressure, and end-organ dysfunction (collapse, syncope, incontinence) [98].
Treatment
The most important step in the management of drug induced urticaria with or without angioedema is withdrawal of the causative agent. In most cases of acute urticaria, when the trigger is removed the rash quickly resolves. H1-receptor blockers are the mainstay of treatment for patients with only cutaneous symptoms. Systemic glucocorticoids are indicated in all cases with upper airway edema and should be considered in cases with extensive cutaneous involvement. Epinephrine is reserved for angioedema with upper airway involvement [94]. The presence or absence of any airway involvement should be specifically investigated.
Prognosis
Both urticaria and angioedema fade without visible sequelae. Following resolution, there should be no residual pigmentary changes unless excoriated [94].
Offending Drugs
Many drugs can induce acute urticaria, and do so by both immunologic and non-immunolgic mechanisms. The major drugs responsible for immunologically based urticaria are antibiotics, especially penicillins and cephalosporins [90]. The major drugs triggering mast cell release (non-immunolgic mechanisms) are aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), opioids and radiocontrast media [90]. Viral infections or connective tissue diseases may induce or augment urticarial drug reactions [86].
Urticarial Eruption – Complex (Skin + Systemic Involvement)
Anaphylaxis
The National Institute of Allergy and Infectious Diseases (NIAID) and the Food Allergy and Anaphylaxis Network (FAAN) defined anaphylaxis as a systemic reaction resulting from the sudden release of multiple mediators from mast cells and basophils, often life threatening, and usually unexpected. The World Allergy Organization (WAO) has divided anaphylaxis into immunologic (further divided into immunoglobulin E [IgE]-mediated and non-IgE-mediated), non-immunologic, and idiopathic causes. Drugs are the second most common cause of anaphylaxis after food, which constitutes 20 % of triggers [98]. Common medications associated with anaphylaxis include penicillins, NSAIDs, and biologic response modifiers [99]. The NIAID/FAAN definition of anaphylaxis has been translated into clinical diagnostic criteria that include an acute onset of illness (minutes to hours) and involvement of the dermatologic, respiratory, cardiovascular, or gastrointestinal systems [98]. Epinephrine is the only first-line treatment for anaphylaxis and is the sole effective treatment for an acute reaction. Delays in administration have been associated with fatalities. Supportive treatment with oxygen, fluids and additional drugs are also necessary according to the cardiopulmonary resuscitation (CPR) anaphylaxis algorithm [98].
Serum sickness-like reaction (SSLR) – See Severe Cutaneous Adverse Drug Reactions.
Pustular Eruptions
Skin Signs
Pustular drug eruptions are characterized by monomorphic eruption consisting of erythematous papules (mostly follicular) and pustules at the same location lacking comedones.
Pustular Eruptions – Simple (Skin Only)
Acneiform Drug Eruptions (Acne Medicamentosa)
The term acneiform is applied to eruptions that resemble acne vulgaris.
Frequency
Varies, depending on the drug. The highest incidence involves epidermal growth factor receptor inhibitors (EGFRIs), affecting 60–100 % of patients [101].
Lag Period
Symptoms
Pruritus, tenderness and pain may occur. In cases of chemotherapy-related side effects, their appearance and severity are part of the criteria used for the classification of the ADR [103].
Common Sites of Involvement
Lesions may be located in and beyond the seborrheic areas, such as the arms, trunk, lower back and genitalia [104].
Histology
Drug-induced acneiform eruptions show histopathologic features similar to acne vulgaris. Early lesions most commonly have a corneocytic plug within a widened infundibulum, accompanied by infundibular spongiosis, perifollicular edema, with sparse perivascular and peri-infundibular infiltrates of neutrophils and lymphocytes. Larger older lesions show similar findings but the infiltrate is denser, with more neutrophils around the involved follicles, and infundibular rupture [85, 88]. In a review of the histological findings of acneiform eruptions induced by EGFRIs [105], all ten cases showed a superficial, predominantly neutrophilic suppurative folliculitis with ectatic infundibula and a rupture of the epithelial lining.
Differential Diagnosis
The main differential diagnosis is acne. The following clinical characteristics of acneiform drug eruptions may aid in differentiating between the two entities: (1) Clinical presentation: monomorphic pattern, lack of comedones and cysts and localization on areas beyond the seborrheic area. (2) Patient characteristics: age of onset before or after the teens, and absence of past history of acne. (3) Resistance to conventional acne therapy. (4) Time relationship: onset after recent drug introduction, improvement after drug withdrawal, and recurrence after drug reintroduction [101]. The differential diagnosis also includes folliculitis, rosacea, perioral dermatitis, demodicosis, acne cosmetic, acne mechanica, chloracne, acne necrotica and acneiform presentation of cutaneous lymphomas [104].
Treatment
The main treatment is withdrawal of the offending drug and the application of topical treatments as needed (benzoyl peroxide topical antibiotics and topical retinoids) [90]. The management of acneiform eruptions associated with chemotherapy differs from all other types of acneiform drug euptions, as acneiform eruption is an expected outcome and discontinuation of the medication is not an option in a patient who is responding to therapy [102, 103, 106, 107]. In fact, continuation of EGFRI therapy in these patients may be especially favourable in view of studies that have shown an increased survival with increasing severity of rash [102]. The cutaneous reaction serves as an important clinical tool for determining tumor response and survival [102]. The National Cancer Institute developed a scale for defining the degree of rash and laid down management guidelines for each stage [103]. Other management protocols were suggested by Bachet et al. [107], who recommended that unless contraindicated, a tetracycline should be routinely prescribed for the prevention of acneiform eruption in patients treated with an EGFRI for more than 6 weeks. Chiang et al. [106] reported successful treatment with isotretinoin for high grade and refractory cases.
Prognosis
In most patients with acneiform drug eruption, the rash resolves upon discontinuation of the offending drug and the use of topical treatment. In EGFRI-induced acneiform eruption, prophylactic administration of a tetracycline was associated with significantly lower incidence of grade 2–3 folliculitis and improved quality of life of patients [107].
Offending Drugs
The drugs responsible for acneiform eruptions include [101]:
Hormones: corticosteroids and corticotropin – adrenocorticotropic hormone (ACTH), androgens and anabolic steroids, hormonal contraceptives; other hormones – thyroid-stimulating hormone, danazol.
Neuropsychotherapeutic drugs: tricyclic antidepressants, lithium, antiepileptic drugs, aripiprazole, selective serotonin reuptake inhibitors.
Vitamins: B1, B6, B12, D2.
Cytostatic drugs: dactinomycin – actinomycin D, azathioprine, thiourea, thiouracil.
Immunomodulating molecules:cyclosporine, sirolimus.
Antituberculosis drugs: isoniazid, rifampin, ethionamide.
Halogens: iodine, bromine, chlorine.
Targeted therapies: EGFRIs (cetuximab, panitumumab), multitargeted tyrosine kinase inhibitors (gefitinib, erlotinib, lapatinib,sorafenib, sunitinib, imatinib), vascular endothelial growth factor inhibitor (bevacizumab), proteasome inhibitor (bortezomib), tumor necrosis factor-a inhibitors (lenalidomide,infliximab), histone deacetylase inhibitor (vorinostat).
Miscellaneous: dantrolene, quinidine, antiretroviral therapy antibiotics.
Drug-Induced Eosinophilic Pustular Folliculitis (Ofuji’s Disease)
Few cases of drug-induced eosinophilic pustular folliculitis have been reported [88, 108–111]. Drugs reported include chemotherapy (cyclophosphamide, methotrexate, and 5-fluorouracil) [108], minocycline [109], carbamazepine [110], and allopurinol with timedium bromide [111]. Clinical presentation includes pruritic follicular papules and pustules on the face, scalp, trunk and arms [88]. Histological findings include spongiosis of the follicular epithelium, and an intra- and perifollicular lymphohistiocytic infiltrate with numerous eosinophils that form microabscesses within the follicular epithelium [88]. Topical steroids are the first line of treatment [108].
Pustular eruptions – Complex (skin + systemic involvement)
Acute generalized exanthematous pustulosis (AGEP) – See Severe Cutaneous Adverse Drug Reactions.
Bullous Eruptions
Bullous Eruptions – Simple (Skin Only)
Pseudoporphyria
Terminology
The term pseudoporphyria was coined in 1975 by Korting to describe patients with chronic renal failure and a bullous disease resembling porphyria cutanea tarda (PCT) [112].
Frequency
The incidence of pseudoporphyria is unknown. However, in a 6-month prospective study, 12 % (9/74) of children taking naproxen for juvenile idiopathic arthritis developed pseudoporphyria [113].
Lag Period
Skin Manifestations
The clinical features of pseudoporphyria may be identical to those of PCT; both exhibit vesicles, bullae, milia, and scarring on sun-exposed skin. In contrast to PCT, however, hypertrichosis, hyperpigmentation, sclerodermoid changes, and dystrophic calcification are rarely reported in pseudoporphyria [117]. Often, fragility and bruising may be the only clinical signs [116]. In children, facial scarring resembling erythropoietic protoporphyria (EPP) may be found [117].
Common Sites of Involvement
The lesions appear on sun-exposed skin, particularly the hands and feet, but also on the face and extensor surfaces of legs [116].
Histology
the histological features are identical to those seen in PCT. The blisters are subepidermal and the floor of the blister is typically lined by well-preserved dermal papillae (festooning). There is usually no significant inflammatory component although a light perivascular lymphocytic infiltrate may occasionally be seen in the superficial dermis. Thickening of the superficial vessels (highlighted by a PAS stain) and dermal sclerosis with elastosis may be apparent. In both pseudoporphyria and PCT, direct immunofluorescence reveals granular deposits of IgG and C3 at the basement membrane zone and in the perivascular region [115].
Differential Diagnosis
While pseudoporphyria and PCT share clinical and histologic features, they can be differentiated by several features. Most important, by definition, biochemical porphyrin abnormalities are absent in pseudoporphyria. Epidemiologically, pseudoporphyria affects mainly women while there is a male predilection in PCT. Clinically, hypertrichosis, hyperpigmentation, sclerodermoid changes, and dystrophic calcification are frequently evident in PCT and conspicuously absent in pseudoporphyria [117]. The differential diagnosis also includes other types of cutaneous porphyria that manifest with blistering, epidermolysis bullosa acquisita, polymorphous light eruption, and other photosensitive dermatosis [117].
Treatment
Treatment entails discontinuation of suspected agents and sun protection, especially against UVA wavelengths, for several months following withdrawal of the drug [114].
Prognosis
Blisters may continue to appear for weeks-months after discontinuation of the offending drug [117].
Fixed Drug Eruption (FDE)
Terminology
Lag Period
After initial use of the offending agent, a variable refractory period of weeks, months or years may pass before the lesions first appear on the skin of a sensitized individual [121]. Repeated exposure to the agent typically results in acute lesions within 30 min to 8 h. A refractory phase may occur following an acute flare in which exposure to the offending drug will not exacerbate the lesion for weeks to months [121].
Skin and Oral Membrane Manifestations
In its classical form, FDE typically presents round or oval, sharply demarcated, red to livid, slightly elevated plaques ranging from several millimeters to over 10 cm in diameter. Vesicles or even blisters can develop [122]. Usually only a single lesion appears. Sometimes, multiple lesions are present and even lead to generalized FDE characterized by multiple, sharply defined, deep red macules distributed bilaterally and often symmetrically. Generalized bullous FDE is characterized by flaccid blisters arising on these macules. Mucosal lesions are usually bullous and may appear with or without involvement of other areas of the skin [122].
Symptoms
Common Sites of Involvement
The eruption can occur anywhere on the body, but the lips, palms, soles, genitalia (especially male genitalia), groin and occasionally oral mucosa are favored sites [121]. The diagnostic hallmark of FDE is the reappearance of the lesions precisely over the previously affected sites. Studies investigating the predilection areas indicate that some specific kind of drugs cause FDE predominantly at specific sites: examples are tetracycline and location on the male genital area, and naproxen and FDE on the lips [122]. In rare cases, FDE manifests in old trauma sites such as BCG vaccination, burn scar, venipuncture site or insect bite. With each recurrence, additional sites may be affected. The presence of numerous lesions is referred to as generalized FDE [122].
Histology
Histologically, the acute phase is characterized by marked basal cell hydropic degeneration, with lymphocyte tagging along the dermoepidermal junction and individual keratinocyte necrosis. Marked pigmentary incontinence is typical, and may be the sole histological finding in late lesions [121].
Differential Diagnosis
Skin lesions can imitate various dermatoses, including lichen planus, erythema multiforme, erythema annulare centrifugum, and pityriasis rosea. In generalized FDE, residual pigmentation in healed lesions may be reminiscent of erythema dyschromicum perstans. Involvement of oral and genital mucosa raises the possibility of herpes simplex, pemphigus vulgaris, aphthous stomatitis, Behçet syndrome, and erosive lichen planus [122]. Generalized bullous FDE may resemble SJS/TEN. The following typical clinical features of generalized bullous FDE may aid in differentiating between conditions: (1) Blistering usually affects only a small percentage of body surface area, and between the large blisters there are sizable areas of intact skin. (2) Erosive mucosal involvement is rare, and when it does occur is rather mild. (3) Patients usually do not feel sick or have fever, and generally are in much better overall health than those with SJS/TEN. (4) Most patients report a history of a similar, often local reaction [123].
Treatment
For mild lesions, topical corticosteroids usually suffice. In severe involvement, especially generalized bullous FDE, systemic corticosteroids may be indicated. Strict avoidance of the causative drug and cross-reacting substances is essential for prophylaxis. Successful desensitization was reported [122].
Prognosis
Offending Drugs
The most common groups of drugs implicated are antibiotics, analgesics, antiphlogistics and hypnotics [122]. There is usually only one causative drug (monosensitivity), but sometimes several drugs can induce FDE in the same patient (multisensitivity). It has also been claimed that recurrences of FDE can be induced in non-specific fashion by mast cell degranulators such as food, acetylsalicylic acid, bacterial toxins, or physical stimuli [122].
Bullous Eruptions – Complex (Skin + Systemic Involvement)
Drug-induced/triggered autoimmune blistering dermatosis (pemphigus, bullous pemphigoid (BP)) and linear IgA bullous dermatosis (LABD)
Terminology
Pemphigus
Two Italian dermatologists, Caccialanza and Bellone, were the first to imply activation of pemphigus by a drug (penicillin) in 1951 [124]. However, Degos’s publication in 1969 of penicillamine-induced pemphigus in a patient with Wilson’s disease is considered the first report of drug-induced pemphigus [125].
Cases of autoimmune blistering dermatosis resulting from exposure to drugs present clinical, histologic and immunopathologic features identical or very similar to those seen in idiopathic disease, but are induced by systemic ingestion or local use of certain drugs. There appear to be two main types: drug-induced autoimmune blistering dermatosis proper, the acute and self-limiting type with rapid resolution after withdrawal of the offending agent; and drug-triggered autoimmune blistering dermatosis in which the role played by the drug is only secondary to hereditary and immunologic factors. The drug stimulates a predisposition (hidden susceptibility) to develop the disease and is considered the chronic type in which the disease persists despite withdrawal of the offending agent [128, 129].
Frequency
Unknown.
Lag Period
Symptoms/Common Sites of Involvement/Histology
Similar to the idiopathic type of autoimmune blistering dermatosis.
Differential Diagnosis
There are no distinctive clinical features that enable differentiation between drug-induced/triggered and idiopathic autoimmune bullous dermatosis. It is obvious that spontaneous remission following withdrawal of the offending drug points to a drug-induced autoimmune blistering dermatosis. However, other clinical findings may also be suggestive of a drug origin in cases of pemphigus and BP: (1) Patients are younger than those with idiopathic disease. (2) Mucous membranes are frequently involved. (3) Combined clinical and immunohistologic features of various immunobullous diseases may exist. (4) Severe general status may appear including high fever. (5) In cases of drug-induced pemphigus, features of pemphigus foliaceus are more common than those of pemphigus vulgaris [130, 133, 136]. Of note, drug-induced LABD patients tend to be older than idopathic type patients [134, 135].
The polymorphic nature of the eruption may mimic other bullous diseases and or drug-induced bullous diseases such as SJS, TEN, and FDE [136].
Treatment
Treatment consists of discontinuing the offending agent, and, depending on the severity of the disease, systemic immunosuppressive treatment [129].
Prognosis
Offending Drugs
Pemphigus
BP
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) – See Severe Cutaneous Adverse Drug Reactions.
Severe Cutaneous Adverse Drug Reactions
Drug reaction with eosinophilia and systemic symptoms (DRESS), Drug-induced hypersensitivity syndrome (DIHS), Drug-induced delayed multiorgan hypersensitivity syndrome.
Epidemiology
The incidence of DRESS remains to be determined because of variable presentations and lack of universally accepted diagnostic criteria [140]. The estimated risk at first or second prescription of an aromatic antiepileptic drug was 1–4.5 in 10,000 [141]. A slight female predominance was found in the RegiSCAR study (male/female 0.8) [142].
Etiology
The drugs most commonly inducing DRESS are anti-convulsants (mainly aromatic anti-convulsants such as carbamazepine), allopurinol, sulfonamides (the anti-infective sulfamethaxazole-trimethoprim, and the anti-inflammatory sulfasalazine), and antibiotics (such as vancomycin and minocycline) [142]. Numerous other drugs have been reported [140, 143, 144].
The role of human herpesvirus (HHV) reactivation in the development of this adverse drug reaction is well recognized, especially HHV-6 [145]. HHV-6 reactivation is among the diagnostic criteria of the Japanese consensus group for DRESS/drug-induced hypersensitivity syndrome [146]. The reactivation of other herpesviruses, including HHV-7, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpes simplex virus was also reported [147].
DRESS is considered to result from complex interactions between genetic predisposition, exposure to drug and viral reactivation [148].
Lag Period
Delayed onset of 2–8 weeks after drug administration followed by a stepwise development of manifestations. Rechallenge can result in a reaction within hours to days [26]. The lag period differs between drugs; carbamazepine tended to show a longer latency (median 29 days) than allopurinol (median 20 days) in the RegiSCAR study [142].
Clinical Features
DRESS has multi-organ involvement with cutaneous, mucosal, hematological and solid organ manifestations.
Skin
The cutaneous involvement in DRESS is typically extensive and symptomatic (pruritus, burning and pain) [142, 143]. Various dermatological features were reported. Walsh et al. [143] proposed a classification system based on four distinct patterns: (1) urticated papular exanthema, the most common, (2) morbilliform erythema, (3) exfoliative erythroderma, and (4) erythema multiforme-like (EM-like), which was prognostic of more severe hepatic involvement. The extent of skin involvement varies between studies: it exceeded 50 % of the body surface area in most of the patients (79 %) according to the RegiSCAR study [142]; head and neck edema observed in most patients [26, 142]; and pustules reported in various studies, predominantly in a facial distribution of the edema [142, 143].
Mucous Membranes
Mild mucosal involvement was recorded in 56 % of patients with DRESS (66/117 cases) in the RegiSCAR study [142]. Most frequent were oral lesions including lips, oral cavity and throat [142]. The manifestations of oral lesions in DRESS include cheilitis, erosions and dysphagia that may appear before skin lesions, and oropharaynx is considered the first site of herpesvirus reactivation in DRESS [149]. Involvement of eyes and genitalia were also reported in the RegiSCAR study [142].
Systemic Involvement
Multi-organ involvement is common in DRESS and may include a wide variety of systems. High-grade fever (38–40 °C) is a typical early manifestation that may last for several weeks; it often precedes the cutaneous eruption by several days [142]. Lymphadenopathy is common and has two distinct types: a benign pattern of lymphoid hyperplasia and a pseudolymphoma pattern [150]. Hematologic abnormalities are frequent and diverse, the most common being marked leukocytosis, eosinophilia and atypical lymphocytes [142]. However, neutrophilia, monocytosis, thrombocytopenia, anemia, pancytopenia and hemophagocytic syndrome were also reported [140, 142, 143, 151]. Hypereosinophilia and activated neutrophils, if persistent, can contribute to organ damage [142]. The liver is the most frequently affected visceral organ in DRESS; hepatitis with isolated elevation of liver enzymes is common and usually anicteric and without cholangitis. However, severe acute hepatitis with liver failure may result and is the primary cause of mortality in DRESS [150]. Renal involvement is common [150]. Involvement of the following organs was also reported: lungs, muscle, heart, pancreas, colon, thyroid, joints, parotid gland and brain [150]. The type of organs involved was found to be related to the eliciting drug [152].
Histology
The most common pathological changes found in a study of 32 patients with DRESS were basket-weave hyperkeratosis (94 %), dyskeratosis (97 %), lymphocytic exocytosis (91 %), spongiosis (78 %), papillary edema (66 %), perivascular lymphocytic infiltration (97 %), eosinophilic infiltration (72 %), and interface vacuolization in the dermoepidermal junction (91 %) [26]. The presence of severe dyskeratosis was correlated with a greater extent of systemic involvement [26]. In a different study assessing the histological findings of 27 cases with DRESS [143], the predominant pathological pattern was spongiotic dermatitis with superficial lymphocytic infiltrate (59 %); necrotic keratinocytes were noted in 33 % of cases, and were associated with a worse hepatic involvement [143].
Diagnostic Criteria
Treatment
The first step in the management is immediate withdrawal of the culprit drug. The treatment is tailored according to the severity and extent of systemic involvement, and the diagnosis of viral reactivation of herpesviruses (mostly HHV-6) [150, 155, 156]. Management protocol for DRESS based on the consensus of experts was designed by the French Society of Dermatology [156], and includes four visceral involvement severity categories and corresponding treatment: (1) No severe systemic involvement: topical corticosteroids (potent or very potent), emollients, H1-antihistamines. (2) Severe systemic involvement (transaminases >5 times normal, renal involvement, pneumonia, hemophagocytosis, cardiac, etc.): systemic corticosteroids equivalent to 1 mg/kg/day of prednisone and multidisciplinary evaluation. (3) Life-threatening signs (hemophagocytosis with bone marrow failure, encephalitis, severe hepatitis, renal failure, and respiratory failure): systemic steroids with intravenous immunoglobulin (IVIG) at a dose of 2 g/kg over 5 days. The IVIG should not be used without associated steroids. The treatments are to be conducted under multidisciplinary supervision. (4) Severe systemic involvement and confirmation of a major viral reactivation: combining steroids and antivirals (such as ganciclovir) and/or IVIG.
Prognosis
Symptoms are usually present for several weeks even after discontinuation of the offending agent and appropriate treatment [155]. Late complications include the appearance of autoimmune diseases such as lupus erythematosus and autoimmune thyroiditis, with laboratory evidence of autoantibodies [144]. Systemic corticosteroids were found beneficial in the prevention of autoimmune disease. However, this effect needs to be counterbalanced against the higher risk of viral reactivation and infection. [144]. In a 1-year follow-up study of 52 affected patients with DRESS in Taiwan, the overall cumulative incidence of long-term sequelae was 11.5 %; four developed autoimmune diseases (Graves disease, type 1 diabetes mellitus and autoimmune hemolytic anemia); and the other two developed renal failure and required lifelong hemodialysis. The author concluded that the sequelae of DRESS can be divided into two major types that appear in different age groups: young patients tend to develop autoimmune diseases; elderly patients are more vulnerable to end-organ failure [158].
Serum Sickness-Like Reaction (SSLR)
Epidemiology
Etiology
Clinical Features
Skin
The skin is the most frequent finding in SSLR, including erythema that progresses to urticarial lesions (pruritic and migratory), urticarial wheals with dusty to purple centers (‘purple urticaria’) that morphologically resemble erythema multiforme (EM) [161] and other cutaneous manifestations including morbilliform or scarlatiniform eruptions [82].
Systemic Involvement
Joint involvement may be prominent, presenting with edema, decreased range of motion, warmth, pain, and difficulty walking. Polyarticular involvement is often observed, with involvement mainly of the wrists, ankles, hips and knees [169]. Some authors suggested that joint involvement may be related in part to increased fluid in the skin around affected joints due to urticarial eruption rather than arthritis [161]. Fever, malaise, myalgia and lymphadenopathy were also reported. Neurologic involvement, gastrointestinal symptoms and renal complications were rarely documented [163]. Notable laboratory abnormalities include elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and leukocytosis [163, 170].
Histology
Diagnostic Criteria
There are no diagnostic criteria. The diagnosis is based on clinical findings [161].
Treatment
Withdrawal of the offending agent and symptomatic treatment with oral antihistamines and topical corticosteroids are usually sufficient. A short course of oral corticosteroids may be required in patients with severe symptoms [82].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree