Itch, or pruritus, is a hallmark feature of atopic dermatitis (AD). The impact of AD-related pruritus can range from mildly distressing or distracting to completely disabling. Traditionally, management of itch in AD patients has focused on restoring the altered skin barrier with topical emollients and/or reducing inflammation. A growing emphasis has been placed on directly targeting the neural transmission pathways that mediate itch signaling. Off-label use of neuromodulatory agents has helped reduce this aggravating symptom in atopic patients. This article reviews the current literature on the use of neuromodulatory agents and nonpharmacologic alternative therapies used to treat AD-related pruritus.
Key points
- •
Itch is a key component of atopic dermatitis (AD) and has a negative impact on patient quality of life.
- •
Reducing itch symptoms is central to disease control and may require a multifaceted approach to patient care, including barrier protection, pathogen reduction, and use of neuromodulators, in addition to standard immunosuppressive strategies.
- •
Currently, existing evidence to support the use of topical and systemic neural-targeted therapies is limited. Thus far, the greatest benefit has been demonstrated for opioid antagonists, topical anesthetics, and systemic antidepressants.
- •
Alternative therapies, such as acupuncture, stress management, and behavioral therapies, may benefit atopic patients in reducing itch.
Introduction
Itch, also referred to as pruritus, is the principal symptom of AD and its presence is essential to making the diagnosis of the disease. The severity of itch in AD ranges from mild to severe based on the degree of inflammation, the extent or site of involvement, and the chronicity of disease. Pruritus can be so intense that patients scratch until they bleed or produce scarring. Chronic itch in AD often precipitates sleep disturbance, attention difficulties, and social withdrawal, all contributing to a decreased quality of life of affected individuals.
Itch sensation is mediated by activation of small-diameter unmyelinated or thinly myelinated nerves, known as C fibers or Aδ fibers, respectively, whose peripheral terminals reside in the skin ( Fig. 1 ). The central projections of these afferent nerve fibers send itch signals to second-order spinal neurons in the dorsal horn of the spinal cord, which in turn project to the ventrocaudal part of the nucleus medialis dorsalis in the thalamus via the contralateral spinothalamic tract and then onto higher cortical areas ; see Fig. 1 ). The sensation of itch is perceived after activation of the somatosensory cortex and a subsequent scratching reflex is generated in the motor cortex and associated motor cortex. The intensity and quality of itch signals may be influenced at various points along the peripheral, spinal, and/or cortical pathways by other ascending inputs from the periphery (eg, other incoming pain or tactile or temperature-evoked sensations) or descending neural circuits (eg, influence of mood and attention). Understanding these modulatory circuits is of particular interest and relevance in atopic itch because neurophysiologic and psychomimetric testing demonstrates that patients with AD exhibit reduced thresholds for itch and alloknesis (the ability of a nonpruritic stimulus to evoke itch) in involved and uninvolved skin.
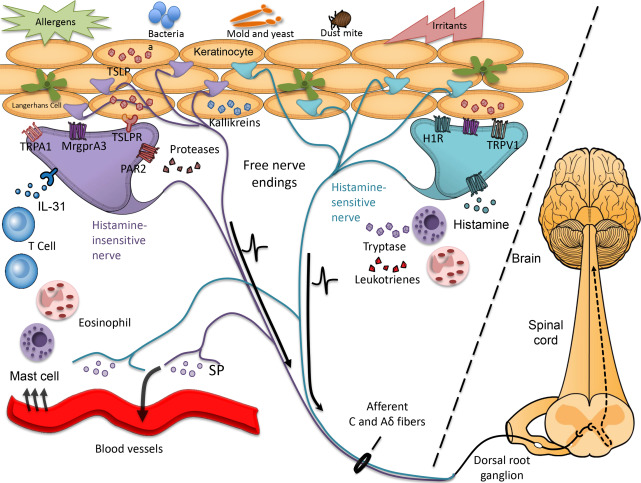
The skin-nerve interface is altered in atopic skin, with several studies demonstrating increased innervation density and expression of inflammatory neuropeptides (eg, substance P [SP], calcitonin gene–related peptide [CGRP], and vasoactive intestinal peptide in lesional atopic skin ; reviewed by Mollanazar and colleagues ; see Fig. 1 ). Numerous exogenous stimuli (eg, irritants and house dust mite allergens) and endogenous factors, including histamine, leukotrienes, cytokines, proteases, neuropeptides, and many other inflammatory molecules, activate cutaneous pruriceptors (itch-sensing fibers). The repertoire of pruritogens is expanded in AD such that nonpruritogenic and/or painful stimuli, including acetylcholine and bradykinin, evoke itch rather than pain. Inflammatory cytokines interleukin (IL)-31 and thymic stromal lymphopoeitin (TSLP), both elevated in atopic skin, are capable of directly activating peripheral nerves to induce itch signaling in animal models. Pathogenic bacteria and yeast, often colonizing atopic skin, may also directly activate peripheral nerves, exacerbating itch symptoms.
Given the complexity of drivers that induce itch (see Fig. 1 ) and growing evidence that supraspinal processing of itch may also be altered in AD patients, the optimal approach to managing itch in AD, particularly in severe cases, must be broad and target multiple points along the itch pathway. Limiting inflammation in the skin via topical or systemic immunosuppressive therapy in patients with active eczema lesions or areas of chronic aggravation is imperative. In addition, all patients should be educated on how best to repair and reinforce the skin barrier in efforts to limit access to infection, irritants, or allergens, all of which stimulate or aggravate itch. Use of topical anesthetics or systemic neuromodulators can dampen itch signaling and may limit neural sensitization when used sufficiently early and used consistently. Moreover, limiting neuronal reflexes that trigger release of SP and other neuropeptides into the skin may reduce vascular dilatation, erythema, and subsequent recruitment of inflammatory cells. Finally, addressing physical and emotional stress is integral to an effective therapeutic regimen.
Introduction
Itch, also referred to as pruritus, is the principal symptom of AD and its presence is essential to making the diagnosis of the disease. The severity of itch in AD ranges from mild to severe based on the degree of inflammation, the extent or site of involvement, and the chronicity of disease. Pruritus can be so intense that patients scratch until they bleed or produce scarring. Chronic itch in AD often precipitates sleep disturbance, attention difficulties, and social withdrawal, all contributing to a decreased quality of life of affected individuals.
Itch sensation is mediated by activation of small-diameter unmyelinated or thinly myelinated nerves, known as C fibers or Aδ fibers, respectively, whose peripheral terminals reside in the skin ( Fig. 1 ). The central projections of these afferent nerve fibers send itch signals to second-order spinal neurons in the dorsal horn of the spinal cord, which in turn project to the ventrocaudal part of the nucleus medialis dorsalis in the thalamus via the contralateral spinothalamic tract and then onto higher cortical areas ; see Fig. 1 ). The sensation of itch is perceived after activation of the somatosensory cortex and a subsequent scratching reflex is generated in the motor cortex and associated motor cortex. The intensity and quality of itch signals may be influenced at various points along the peripheral, spinal, and/or cortical pathways by other ascending inputs from the periphery (eg, other incoming pain or tactile or temperature-evoked sensations) or descending neural circuits (eg, influence of mood and attention). Understanding these modulatory circuits is of particular interest and relevance in atopic itch because neurophysiologic and psychomimetric testing demonstrates that patients with AD exhibit reduced thresholds for itch and alloknesis (the ability of a nonpruritic stimulus to evoke itch) in involved and uninvolved skin.
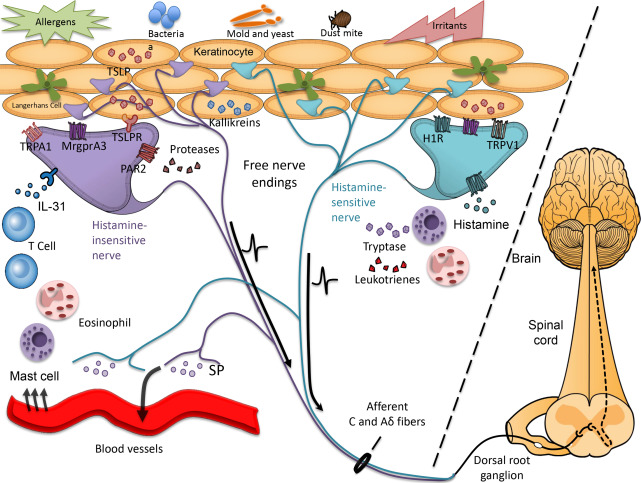
The skin-nerve interface is altered in atopic skin, with several studies demonstrating increased innervation density and expression of inflammatory neuropeptides (eg, substance P [SP], calcitonin gene–related peptide [CGRP], and vasoactive intestinal peptide in lesional atopic skin ; reviewed by Mollanazar and colleagues ; see Fig. 1 ). Numerous exogenous stimuli (eg, irritants and house dust mite allergens) and endogenous factors, including histamine, leukotrienes, cytokines, proteases, neuropeptides, and many other inflammatory molecules, activate cutaneous pruriceptors (itch-sensing fibers). The repertoire of pruritogens is expanded in AD such that nonpruritogenic and/or painful stimuli, including acetylcholine and bradykinin, evoke itch rather than pain. Inflammatory cytokines interleukin (IL)-31 and thymic stromal lymphopoeitin (TSLP), both elevated in atopic skin, are capable of directly activating peripheral nerves to induce itch signaling in animal models. Pathogenic bacteria and yeast, often colonizing atopic skin, may also directly activate peripheral nerves, exacerbating itch symptoms.
Given the complexity of drivers that induce itch (see Fig. 1 ) and growing evidence that supraspinal processing of itch may also be altered in AD patients, the optimal approach to managing itch in AD, particularly in severe cases, must be broad and target multiple points along the itch pathway. Limiting inflammation in the skin via topical or systemic immunosuppressive therapy in patients with active eczema lesions or areas of chronic aggravation is imperative. In addition, all patients should be educated on how best to repair and reinforce the skin barrier in efforts to limit access to infection, irritants, or allergens, all of which stimulate or aggravate itch. Use of topical anesthetics or systemic neuromodulators can dampen itch signaling and may limit neural sensitization when used sufficiently early and used consistently. Moreover, limiting neuronal reflexes that trigger release of SP and other neuropeptides into the skin may reduce vascular dilatation, erythema, and subsequent recruitment of inflammatory cells. Finally, addressing physical and emotional stress is integral to an effective therapeutic regimen.
Immunosuppressive strategies
Reducing inflammatory mediators in the skin, some of which are direct pruritogens, is critical to reducing pruritus and achieving disease control in AD. The specific immunosuppressive regimen is usually determined by the severity and distribution of eczema involvement or pruritus. When localized or mild in severity, topical agents may be sufficient to control AD-related itch. Once generalized or moderate in severity, total skin or systemic therapies may be better suited to manage symptoms. Data supporting the use of these different therapies with respect to atopic itch are briefly reviewed later (summarized in Tables 1 and 2 ). A more thorough discussion of these agents and their use in AD is included in other articles in this issue.
| Therapy | Mechanism of Action | Supporting Evidence in Atopic Dermatitis–Related Itch | Comments |
|---|---|---|---|
| Moisturizers | |||
| Occlusive agents (eg, petrolatum, mineral oil, dimethicone) | Prevent water evaporation | A | First-line therapy |
| Humectants (eg, glycerol, lactate[s], urea, sorbital) | Attract and hold water | B | — |
| Emollients (eg, glycol stearate, glyceryl stearate, lanolin) | Lubricate and soften the skin | A | First-line therapy |
| Repair creams | Lipid and ceramide incorporation into corneocyte scaffold | B | — |
| Topical immunomodulators | |||
| Corticosteroids (eg, hydrocortisone, triamcinolone, betamethasone) | Activates glucocorticoid receptors that inhibit proinflammatory cytokine release | A | First-line therapy; use may be limited by risks of skin-thinning, observed with prolonged use |
| Calcineurin inhibitors (eg, tacrolimus, pimecrolimus) | Prevents activation of the NFAT transcription factor in T cells, thereby inhibiting T-cell activation and proinflammatory cytokine release | A | First-line maintenance |
| Antihistamines (eg, diphenhydramine, doxepin) | Blocks H1 and/or H2 receptors on histamine-sensitive nerve fibers | B | May cause sedation with systemic absorption May cause allergic contact dermatitis |
| Topical antibiotics | |||
| Antibacterial agents (eg, bacitracin, neosporin, mupirocin) | Reduces bacterial colonization, infection, and release of exotoxins | B | Cochrane review failed to show benefit in AD patients unless infected |
| Topical neurmodulators | |||
| Anesthetics | |||
| Caine anesthetics (eg, lidocaine, prilocaine) | Inhibit voltage-gated sodium channels and thereby reduce the firing of nociceptive and pruriceptive sensory fibers | D | Risk of allergic sensitization Prilocaine associated with methemoglobinemia in pediatric patients |
| Polidocanol 3% | Nonionic surfactant | B | — |
| TRP modulators | |||
| Capsaicin | Activates TRPV1 on sensory fibers, depleting SP over time and prevents neural transmission | B: OLS showed reduction in itch in AD-related prurigo nodularis, but otherwise controversial as antipruritic agent in AD | Burning sensation with initial use may limit patient compliance. Open label study showed reduction in itch in AD-related prurigo nodularis, but otherwise controversial as antipruritic agent in AD. |
| Menthol | Activates TRPM8 on sensory fibers triggering a cooling sensation | C | May be useful in patients who report cooling alleviates symptoms |
| Cannabinoids | |||
| N-PEA cream | Binds CB2 receptor and inhibits breakdown of anandamide; reduces mast cell degranulation | B | — |
| Opioid antagonists | |||
| Naltrexone 1% cream | MOR antagonist | B | — |
| Miscellaneous | |||
| Coal tar | Mechanism unknown | B | Second-line or adjunctive therapy |
| Therapy | Mechanism of Action | Supporting Evidence in Atopic Dermatitis–Related Itch | Comments |
|---|---|---|---|
| Antihistamines | |||
| Sedating antihistamines (eg, doxepin, hydroxyzine) | Blocks H1 and/or H2 receptors on histamine-sensitive nerve fibers | B | Improve sleep quality in AD due to sedating effects, but no improvement in itch |
| Nonsedating antihistamines (eg, fexofenadine, cetirizine) | Blocks H1 and/or H2 receptors on histamine-sensitive nerve fibers | C | Despite common practice, a meta-analysis concluded nonsedating AH were ineffective |
| Immunosuppressive agents | |||
| Cyclosporine | Binds to the intracellular receptor cyclophilin, leading to decreased T-cell activation and transcription of IL-2, a mediator of pruritus | A | Limited by need for shorter duration of use |
| AZA | Purine analog and synthesis inhibitor, limits T-cell and B-cell proliferation | A | Second-line therapy |
| MMF | Inosine monophosphate dehydrogenase inhibitor, T-cell and B-cell proliferation | B | Third-line therapy |
| MTX | Inhibitor of dihyrdofolate reductase, which indirectly inhibits purine synthesis, limits T-cell proliferation | B | Third-line therapy |
| Monoclonal antibodies | |||
| Dupilumab | Humanized mAb that binds to and blocks IL-4 and IL-13 signaling | A | Fast-tracked for FDA approval |
| Omalizumab | Humanized mAb that binds and sequesters free IgE | B | Useful in urticaria and asthma |
| Neuromodulators | |||
| Antiepileptics (eg, gabapentin, pregabalin) | Inhibition of voltage-gated calcium channels in peripheral and spinal nerves | E | May be sedating |
| SSRIs (eg, fluvoxamine, paroxetine, sertraline) | Prevent the reuptake of serotonin into presynaptic terminals, indirectly modulating serotonergic signaling | B | May be sedating |
| SNRIs (eg, mirtazapine) | A serotonin and norepinephrine antidepressant | B | Less anticholinergic SE compared with SSRIs; increased risk of weight gain |
| Aprepitant | Neurokinin-1 receptor antagonist, blocks some actions of SP | E | Not FDA approved for itch |
| μ-Opioid antagonists (eg, naltrexone, nalmafene, naloxone) | μ -receptor antagonism reduces itch sensation | B | — |
| κ-Opioid agonists (eg, butrophanol, nalfurafine) | k-receptor agonism reduces itch sensation | E | Nalfurafine is not available in US, used primarily in Japan |
Topical Corticosteroids
Topical corticosteroids (CSTs) have been the backbone of AD management for half a century and remain first-line therapy for acute eczema flares. Although their efficacy in treating eczema is well documented, only a handful of studies have addressed their ability to alleviate atopic itch. In a meta-analysis of 83 randomized clinical trials (RCTs) evaluating the use of topical CSTs in AD, only 39 studies investigated their effect on pruritus. Of these, only 7 trials addressed itch as an independent metric; the other 32 studies included itch within the context of composite severity scores. When compared with placebo, high-potency (class 1) topical CSTs significantly improve atopic itch, often within 1 day to 4 days. A 2012 systematic review of 6 RCTs concluded that atopic itch was reduced by approximately 34% when treated with topical CSTs of varying potencies. Data regarding whether antipruritic efficacy of topical CSTs correlates with greater potency are conflicting, although in many of these reports comparisons were made between closely related potency classes.
Although the data overwhelmingly suggest that topical CSTs significantly improve skin lesions and pruritus in AD patients, the use of these agents is often limited due to the risk of local side effects, including atrophy and the possibility of tachyphylaxis. Due to these limitations and the frequent inability of topicals CSTs to offer complete relief to moderate to severe AD patients, alternative and systemic therapies must frequently be considered.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs), including tacrolimus and pimecrolimus, were introduced more than 15 years ago as steroid-sparing topical agents. Calcineurin inhibitors are immunomodulators that regulate T-cell activation to inhibit the release of inflammatory cytokines. Beyond their anti-inflammatory properties, TCIs may also mediate a direct antipruritic effect by overstimulation and subsequent desensitization of transient receptor potential (TRP) V1 ion channels on cutaneous nerve fibers. In several large, double-blind, placebo-controlled RCTs in pediatric AD patients, tacrolimus ointment alleviated pruritus within the first week of treatment. Similarly, pimecrolimus cream 1% has been shown to reduce itch within days and produces a sustained itch relief with continued use in adult and pediatric AD patients. In several head-to-head studies, tacrolimus ointment was more effective than pimecrolimus cream in the treatment of AD, including AD-related pruritus.
One of the most commonly reported side effects of TCIs includes a burning sensation, in part related to TRPV1 activation on cutaneous nerves. TCIs do not cause skin atrophy and can be safely applied for prolonged periods on any site. Importantly, chronic TCI therapy in AD patients does not increase the risk of systemic immunosuppression, serious infections, or malignancy.
Systemic Immunosuppressive Agents
Cyclosporin A
Cyclosporin A (CsA), an immunosuppressive agent used in transplant medicine since the 1970s, has proved helpful in managing several immune-mediated skin diseases, including graft-vs-host disease, psoriasis, and refractory AD. Cyclosporine binds to the intracellular receptor cyclophilin, leading to decreased T-cell activation and transcription of IL-2, a mediator of pruritus. Although not Food and Drug Administration (FDA) approved for the treatment of AD in the United States, it is the only immunosuppressant approved for AD in Europe. A meta-analysis of CsA efficacy in severe AD patients determined that the mean clinical improvement in disease severity after 6 weeks to 8 weeks of continuous treatment was 55%, although 50% of patients relapsed within 2 weeks. Three of 15 studies included in this meta-analysis were RCTs that included itch severity as a clinical endpoint and demonstrated improvement in pruritus. Additional studies have confirmed reduction in AD-related itch by 71% to 78% when CsA is dosed at 3 mg/kg to 5 mg/kg and by 50% to 54% when dosed independent of body weight at 150 mg or 300 mg daily. Careful consideration must be given to the use and duration of CsA therapy in AD in light of potential serious side effects (for example, hypertension, renal dysfunction, and hyperlipidemia. Thus, CsA may best be used as a short-term solution to achieve rapid control of inflammation and itch in severe AD.
Purine synthesis inhibitors
Mycophenolate mofetil (MMF) inhibits inosine monophosphate dehydrogenase, an enzyme necessary for purine biosynthesis, and thereby prevents lymphocyte proliferation. MMF has been shown to be safe, well tolerated, and effective for the treatment of severe AD and AD-associated itch. In 2011, Haeck and colleagues reported results from an open-label trial in which 55 adult subjects with severe AD were treated with CsA for 6 weeks and subsequently randomized to either continuing CsA or transitioning to MMF for 30 weeks. Although the initial AD severity measurements were better for patients who remained on CsA, eczema severity was similar between MMF and CsA groups after 10 weeks and the duration of clinical remission after discontinuation of drug lasted longer for patients treated with MMF.
Azathioprine (AZA) is a purine analog that inhibits normal purine synthesis and DNA production, limiting T-cell and B-cell proliferation. A 2014 meta-analysis of systemic immunomodulators in the treatment of moderate to severe AD recommended AZA as a second-line treatment option. Several RCTs demonstrated improvement in atopic itch after treatment with AZA. One double-blind RCT of 63 AD patients reported a 2.4-mm reduction on an itch visual analog scale (VAS) in subjects receiving AZA compared with 1-mm reduction in subjects receiving placebo at 12 weeks. Although the potential for lymphopenia, nausea, or gastrointestinal upset must be monitored in patients taking AZA, several studies failed to report serious adverse events after 24 weeks of treatment.
Methotrexate (MTX), an inhibitor of dihyrdofolate reductase that indirectly inhibits purine synthesis, is commonly used in the treatment of inflammatory skin disease and has been recommended as a third-line agent for moderate to severe AD. To date, there are few studies that address the impact of MTX on atopic itch. A prospective, open-label trial evaluating the efficacy of MTX in moderate to severe AD demonstrated a 52% reduction of disease severity from baseline, accompanied by significant improvements in itch severity and sleep. In a head-to-head comparison of AZA and MTX, similar improvement in AD was noted in both groups, with mean decreases in itch on VAS by 2.6 mm (SD 2.2 mm) in patients receiving AZA and 2.5 mm (SD 2.2 mm) in patients receiving MTX.
Monoclonal antibodies
Monoclonal antibody (mAb) therapies may also be used for the treatment of AD. Dupilumab, a fully humanized mAb that binds to and blocks IL-4 and IL-13 signaling, limits key mediators of type 2 helper T-cell allergic inflammation. In a 2013 double-blind, placebo-controlled RCT of adults with moderate to severe AD, dupilumab treatment resulted in significant and rapid improvement in all measured indicators of AD disease severity, including a 55.7% reduction in itch severity compared with only 15.1% in the control group. A subsequent phase 2b dose-ranging study of dupilumab recapitulated these results. Dupilumab has been fast-tracked for approval by the FDA for moderate to severe AD refractory to topical and/or other systemic medications and/or for whom such options are contraindicated. Omalizumab, a humanized mAb that sequesters free IgE, has been approved for the treatment of patients 12 years and older with moderate to severe allergic asthma and chronic urticaria. Although a handful of case reports and small open-label studies suggest a beneficial role in AD, results have been inconsistent and 1 study suggests that this variability may reflect whether or not patients carry filaggrin mutations. The role for other therapies, including anti–IL-5 therapy (mepolizumab), anti-CD20 (rituximab), and immunoabsorption of IgE, in AD is unknown.
Phototherapy
Phototherapy is an effective, safe, and well-tolerated treatment of AD and atopic itch. Although the mechanism of action of phototherapy is incompletely understood, its efficacy in treating atopic pruritus is likely multifactorial. Phototherapy reduces cutaneous inflammation by decreasing T cells in atopic skin, reducing IgE-binding and mast cells in the dermis, and inhibiting the egress of Langerhans cells out of the epidermis. Phototherapy also reduces the density of epidermal sensory nerves, which may contribute to a more direct antipuritic effect. AD and itch are highly responsive to phototherapy regimens that span a broad range of wavelengths, including broadband and narrowband UV-B, combined UV-A + UV-B, and UV-A alone. Although therapeutic benefits, including rapid reduction in itch, have been observed in AD patients after psoralen plus UV-A treatment, potential side effects of erythema, burning, more rapid photoaging, and the risk of nonmelanoma skin cancer limit the use of this option.
Emollients/skin barrier protection
Atopic skin is characterized by a reduction in stratum corneum lipids (eg, ceramides and cholesterol) and/or filaggrin deficiency, both of which result in heightened transepidermal water loss (TEWL), and a dysfunctional skin barrier easily penetrated by irritants, allergens, and pathogens, all of which drive atopic itch and inflammation. Topical moisturizers, comprised of emollient, occlusive, and/or humectant ingredients, improve atopic itch. Emollients (eg, glycol, glyceryl stearate, lanolin, and soy sterols) lubricate and soften the skin; occlusive agents (eg, petrolatum, dimethicone, and mineral oil) form a layer to prevent water evaporation; and humectants (eg, glycerol, lactic acid, and urea) attract and hold water. These ingredients work synergistically to restore the integrity of the epidermal barrier and, when used in combination with topical CSTs, improve the barrier, and reduce itch far better than topical CSTs alone.
Adjunct agents routinely found in moisturizers intended to alleviate itch include colloidal oatmeal and ceramides. Oatmeal has been used for centuries to relieve itch because they contain a group of phenolic alkaloids that decrease production of inflammatory mediators, including arachidonic acid, phospholipase A2, tumor necrosis factor α ; inhibit the activity of nuclear factor κB, a transcription factor important in innate and adaptive immune responses ; and prevent keratinocyte release of proinflammatory cytokines (eg, IL-8) and histamine. In a recent review of studies of adjunct therapies in AD, the daily use of moisturizers and cleansers containing colloidal oatmeal significantly improved clinical outcomes, including itch severity.
Ceramides, a family of waxy lipid molecules that contribute to sphingomyelin, comprise a major component of the intercellular lipids that surround keratinocytes in the stratum corneum and prevent water loss. Decreased ceramide levels in the skin of AD patients contribute to impaired barrier function. In a recent cohort study, a ceramide containing cleanser and moisturizer substantially improved overall disease severity and itch intensity in AD patients. Consistent with this observation, moisturizers with equimolar ratio of cholesterol, ceramides, and essential and nonessential free fatty acids accelerate barrier recovery. These ingredients have recently been incorporated into nonsteroidal barrier creams that integrate directly into the extracellular lipid matrix of the stratum corneum to relieve itch in adult and pediatric patients.
Given the numerous studies that have documented the antipruritic effects of moisturizers, their liberal and frequent use should be a cornerstone of AD therapy. Patients should be directed to apply moisturizers 1 time to 3 times daily, within minutes of bathing for optimal occlusion of a hydrated stratum corneum. The choice of moisturizing agent is highly dependent on individual preference, although preparations devoid of additives, fragrances, and perfumes are recommended. In addition, lower pH moisturizers are preferred in preserving barrier function and in reducing itch and irritation.
Antibacterial agents
Due to a compromised barrier, atopic skin is predisposed to develop secondary bacterial, viral, and fungal skin infections. Staphylococcus aureus is the most common offender, reported to colonize more than 90% of adult AD patients. Staphylococcus-derived exotoxins and superantigens aggravate AD symptoms and are capable of directly triggering itch, and the density of S aureus colonization correlates with disease severity. Although data regarding the utility of topical and systemic antibiotics to control colonization in AD have been controversial (addressed in a recent Cochrane review ) the regular practice of dilute bleach baths with concomitant intranasal topical mupirocin may be helpful for improving AD-related itch. In an RCT of 31 children with moderate to severe AD, treatment of an infectious episode with 2 weeks of oral cephalexin followed by the addition of household bleach (sodium hypochlorite) to bathwater plus intranasal mupirocin treatment for 3 months led to a statistically significant improvement in disease severity compared with placebo. A prospective RCT in Malaysia showed similar results, reporting a significant reduction in itch severity after 2 months of dilute bleach baths. Patients with severe itch may initially complain of irritation due to the weakly basic pH of hypochlorite in water, but this usually abates with subsequent treatments. One additional advantage of bleach over antibiotics is the lower concern for development of bacterial resistance.
Antihistamines
Histamine is a well-established pruritogen and is known to mediate the inflammatory effects of several allergic skin diseases. As such, topical and oral histamines are routinely recommended by physicians to help control atopic itch. Despite this widespread practice, histamine’s role in triggering itch in AD is limited and evidence supporting the use of histamine 1 (H1) or histamine 2 (H2) receptor antagonists in AD management is lacking. Emerging evidence suggests that the H4 receptor may play an important role in mediating histamine-induced inflammation and itch in AD; however, H4 antagonists are not commercially available at present.
Several RCTs evaluating the effects of sedating and nonsedating oral H1 antagonists on itch relief in AD do not support substantial improvement over placebo. A meta-analysis of 16 RCTs concluded that nonsedating antihistamines are ineffective in AD management, whereas sedating forms may improve sleep quality. In the Early Treatment of the Atopic Child trial, infants were randomized to receive cetirizine or placebo for 18 months. Although subjects receiving cetirizine had less urticaria during the treatment period, no statistically significant improvement was observed. A dose-titrating study of 178 adults showed that a 4-fold increase in the dose of cetirizine was required to appreciably reduce pruritus, erythema, lichenification, and body surface area involvement. These findings were attributed to the sedating effects of cetirizine at high doses. A small, but significant, antipruritic effect of fexofenadine, 60 mg twice daily, has been described.
These data support the intermittent use of sedating antihistamines, particularly in the setting of sleep loss secondary to itch. Diphenhydramine and hydroxyzine are the most commonly used antihistamines for this purpose, although some patients become tolerant to the sedating effects within 4 days to 7 days of therapy. Doxepin, a tricyclic antidepressant with potent H1 receptor antagonism, is often chosen as an adjunctive agent in the management of AD because of its combined anxiolytic, antidepressant, and sedative effects. A double-blind RCT in 270 patients demonstrated significant reduction in itch after using topical doxepin 5% cream for 7 days compared with vehicle. Its use, however, is limited by a high rate of allergic contact dermatitis and sedation from percutaneous absorption. Both topical and oral antihistamines may cause sedation (including nonsedating formulations) and may elicit anticholinergic symptoms (eg, dry mouth, blurred vision, and tachycardia).
Neuromodulators
Consistent with the concept of AD being “the itch that rashes,” a growing number of studies suggest that the nervous system is integral to promoting atopic disease in addition to mediating itch. Evolving preclinical data suggest that effectively targeting the neural circuits involved in itch transmission may improve the pruritus, burning, and discomfort from which AD patients suffer. The use of neuromodulators in AD is in its relative infancy and formal evaluation of the efficacy of neuromodulation in double-blind RCTs has been rare. Despite this, preclinical and early clinical data are promising and this field will likely undergo dramatic expansion in the near future.
Topical Neuromodulators
Topical capsaicin
Capsaicin, a naturally occurring alkaloid derived from chili peppers, has been successfully used to relieve pruritus in several conditions, including prurigo nodularis, both AD-dependent and independent cases; neuropathic dermatoses; and aquagenic and uremic pruritus. Capsaicin activates TRPV1 ion channels on nociceptors, triggering the release and depletion of neuropeptides, such as SP and/or CGRP. Although repeated topical application of capsaicin can reduce or eliminate itch in AD-related prurigo nodularis, clear evidence supporting the use of capsaicin as an antipruritic in other stages of AD is lacking. One study actually demonstrated that although pretreatment with capsaicin suppressed histamine-induced itch in healthy controls, it had no effect in reducing itch in atopic individuals. A potential barrier to use, capsaicin causes transient burning and local irritation. This side effect may be mitigated by preceding or concurrent application of topical anesthetics to reduce pain as well as topical CSTs to reduce initial inflammation.
Topical cooling agents
Topical cooling agents have been found helpful in the management of itch and may be of use in mild AD and atopic itch. Cooling agents include menthol, camphor, and phenol, all commonly found additives in topical anti-itch lotions. Menthol and camphor are monoterpenoids that have been used since antiquity and exert their effects via activation of thermosensitive TRP channels expressed by keratinocytes and peripheral nociceptors. In vitro studies demonstrate that camphor’s ability to enhance the sensation of innocuous cold may be due to activation of thermosensitive TRPV3 receptors and ultimately to receptor desensitization that leads to analgesic and antipruritic effects. Similarly, menthol functions as an agonist of TRP melastatin-8 (TRPM8), a temperature-sensitive calcium channel expressed by peripheral afferent fibers. When activated, TRPM8 evokes the sensation of cold and can modulate the perception of pain and itch.
Despite their widespread use in over-the-counter anti-itch preparations, evidence regarding the efficacy of these coolants in reducing atopic itch is sparse. Menthol has been demonstrated to reduce itch in patients with lichen amyloidosis and histamine-evoked and irritant-induced pruritus. One study performed in 35 atopic pediatric patients reported that menthol spray decreased itch from baseline after 7 days. Further evaluation of the efficacy of menthol and similar compounds to treat atopic itch is warranted. Caution must be advised when recommending the use of cooling agents in atopic individuals, because menthol and camphor may exacerbate TEWL. Furthermore, monoterpenes may induce allergic or irritant contact dermatitis and at higher concentrations (10%–40%) may cause erythema and burning, which is of particular concern in AD patients given their compromised skin barrier.
Topical anesthetics
Topical ‘caine’ anesthetics have been effectively used to provide itch relief in several skin conditions. Lidoocaine and prilocaine are both amide anesthetics that inhibit voltage-gated sodium channels and thereby reduce the firing of nociceptive and pruriceptive sensory fibers. In a study in 20 healthy volunteers, a mixture of lidocaine and prilocaine reduced itch evoked by histamine as well as cowhage and papain, experimental pruritogens that activate histamine-independent itch pathways. Although some benefit of topical lidocaine has been demonstrated for managing neuropathic and postburn pruritus, its efficacy in AD is limited to an isolated report in which 1 atopic patient-derived tremendous itch relief after lidocaine application. Allergic sensitization to topical lidocaine must be considered, because the rate of delayed type hypersensitivity reactions is higher in prone populations.
In contrast, the anesthetic surfactant polidocanol, recently shown to reduce histamine-independent itch in an experimental setting by more than 58%, has shown some promise in reducing atopic itch. In a German multicenter, open-label trial in 1611 pediatric and adult patients with AD (47.9%), contact or other eczemas (33.7%), psoriasis (6.2%), or other pruritic dermatoses, a cream mixture of 3% polidocanol and 5% urea improved or entirely eliminated pruritus in the majority of patients. Patients tolerated the topical mixture well overall, infrequently experiencing burning or itch with initial application.
Topical naltrexone
Naltrexone, a μ-opioid receptor (MOR) antagonist, has been shown to reduce atopic itch in several clinical studies. In a pilot study of 18 patients with chronic pruritus of varying etiologies, including AD, more than 70% of the patients using topical 1% naltrexone cream experienced significant itch relief. In a subsequent, randomized, crossover trial in AD patients, topical application of naltrexone resulted in 29.4% improvement in itch and a faster time to relief/itch reduction compared with placebo alone. Skin biopsies from patients using topical naltrexone exhibited decreased MOR expression. Naltrexone and other MOR antagonists, as well as κ-opioid receptor (KOR) agonists, have been reported effective in managing intractable pruritus from multiple sources and are discussed later.
Topical cannabinoids
Palmitoylethanolamine (PEA) is an endogenous fatty acid amide with endocannabinoid-like properties that has antipruritic and analgesic effects. PEA has low affinity for cannabinoid receptors and is thought to exert its anti-itch effects by inhibiting the breakdown of another potent endocannabinoid, anandamide and via reduction of mast cell degranulation. Although double-blind RCTs evaluating topical cannabinoids are not available, a multicenter prospective cohort study of 2456 patients reported that PEA 0.3% cream improved pruritus as well as other symptoms, including lichenification, excoriation, scaling, and erythema, in mild-to-moderate AD patients. Daily use of PEA cream was associated with a 45.6% reduction in VAS score for itch 6 days after beginning treatment and with a 60% decrease after 6 weeks. In this study and in the author’s experience, PEA creams are tolerated well with limited side effect, including occasional burning, itch, or mild erythema, usually limited to 1 to 2 applications (unpublished observations).
Topical coal tar
Although the precise mechanism of action is unclear, topical formulations of crude and refined coal tar have been used for decades to treat inflammatory skin conditions, including AD. Few studies evaluate the anti-inflammatory and antipruritic effects of coal tar in conditions other than psoriasis ; however 1 study demonstrated improvement in AD severity and itch in 18 subjects with mild to moderate AD after treatment with crude coal tar in a zinc paste at least 2 times to 3 times weekly. An open-label comparative study found that a purified coal tar cream was as effective at reducing eczema and pruritus as 1% hydrocortisone cream. Goeckerman therapy, which combines the use of crude coal tar and phototherapy, has also been used for decades to effectively reduce itch and inflammation in atopic patients with severe involvement. Because it is well tolerated, safe, and cost-effective, coal tar has been suggested as a helpful, second-line, or adjunctive agent for AD management.
Systemic Neuromodulators
Antiepileptics
Gabapentin and its prodrug pregabalin, analogs of the inhibitory neurotransmitter γ-aminobutyric acid, play a growing role in the management of neuropathic conditions including chronic pain and itch. Their ability to reduce nociception is attributed to inhibition of voltage-gated sodium and calcium channels in dorsal root ganglia and spinal cord, respectively ; reduction in glutamate synthesis and release ; and decreased neural expression of SP and CGRP, both of which are increased in AD.
Although the efficacy of neuroleptics in managing atopic itch has yet to be formally evaluated, numerous case reports, small case series, and placebo-controlled double-blind RCTs support a role for these agents in the management of other itchy dermatoses, including prurigo nodularis, brachioradial pruritus, notalgia paresthetica, burn-related itch, postherpetic itch, cholestatic itch, and uremic itch. Although sedating effects can limit the rate of dose escalation during the day, gabapentin may be particularly helpful in reducing nocturnal pruritus and scratching in atopic patients. Due to myriad potential side effects, including fatigue, dizziness, blurred vision, nausea, dry mouth, and weight gain, it is prudent to start therapy at low doses (100–300 mg at bedtime) and slowly titrate upwards as tolerated.
Antidepressants
Serotonin and norepinephrine antidepressants
Selective serotonin reuptake inhibitors (SSRIs) block the reuptake of serotonin into presynaptic terminals, thereby increasing the effective concentration of serotonin in synaptic clefts and indirectly modulating presynaptic and postsynaptic serotonin receptors. Serotonin has pleiotropic effects in the skin, reflecting the widespread distribution of serotonin receptors on different cutaneous cell types, including peripheral sensory nerves, keratinocytes, mast cells, lymphocytes, natural killer cells, and Langerhans cells, among others. In animal models, activation of serotonin 7 receptors on peripheral nerves mediates acute and chronic itch signals. SSRIs have shown promise in reducing AD-related itch and prurigo nodularis as well as psychogenic pruritus, polycythemia vera, and cholestatic and paraneoplastic pruritus. In a prospective, open-label trial comparing 2 SSRIs, both paroxetine and fluvoxamine decreased itch severity by approximal 50% in patients with chronic itch, including the subset of patients with AD. Potential side effects of SSRIs include insomnia, weight loss, nausea, fatigue, and changes in sexual libido or function.
Mirtazapine, a serotonin and norepinephrine antidepressant, has been reported to reduce pruritus in AD as well as other chronic pruritic dermatoses. Mirtazapine also exerts anxiolytic and antihistamine effects and the resultant sedative properties may well contribute to its antipruritic actions. Although mirtazapine tends to exhibit fewer anticholinergic effects than SSRIs, users face greater challenges with sedation and weight gain. With all the medications in these classes, it is critical to taper patients off therapy slowly to avoid systemic rebound effects.
Aprepitant
Aprepitant is an FDA-approved antiemetic drug that inhibits the effects of SP on neurokinin-1 receptors in the central and peripheral nervous systems, including cutaneous nerve endings. Although aprepitant has been reported effective in reducing itch in patients with prurigo nodularis and in Sézary syndrome in Europe, reports of its use elsewhere have failed to demonstrate improvement in itch in these conditions and it is has not yet been formally evaluated in AD.
Opioid modulators
Opioids modulate the perception of pain and itch, exerting diverse actions, depending on the receptor activated (MORs, KORs, and δ-opioid receptors) and the site of action within the central and peripheral nervous systems. Naltrexone and nalmefene, MOR antagonists, have been evaluated in the management of AD-related pruritus with promising results. One double-blind RCT in AD and urticarial patients demonstrated that nalmefene significantly reduced or completely eliminated itch compared with placebo. Several double-blind RCTs and open-label prospective trials demonstrate that naltrexone effectively reduces itch severity in atopic patients, in some cases within 2 weeks. Moreover, oral naltrexone was found to reduce acute itch and alloknesis in AD patients. Although a few studies failed to show improvement in atopic itch, the current evidence generally supports the use of MOR antagonists as second-line or adjunctive agents in managing AD-related pruritus. MOR antagonists frequently trigger dose-dependent side effects, including dizziness, fatigue, gastrointestinal upset, or cramping. To limit these effects, initiation of therapy with this class of medications should start with low doses (eg, nalmefene, 10 mg daily, and naltrexone, 25 mg daily) with a slow titration up over the course of weeks.
Animal and human studies suggest that opioid activity in the periphery and spinal cord are critical modulators of itch transmission pathways and that an imbalance in endogenous μ-opioid and κ-opioid activity may result in certain types of chronic itch. Consistent with these observations, nalfurafine, a selective KOR agonist, is approved to treat uremic itch in Japan, but has not been formally tested in AD. Butorphanol, a combined MOR antagonist and KOR agonist, has been reported to benefit patients with intractable itch in the setting of primary biliary cirrhosis, paraneoplastic itch, and senile pruritus. In addition to potential peripheral and spinal antagonism of itch pathways, recent functional neuroimaging studies found that butorphanol caused bilateral deactivation of neural structures involved in processing cowhage-induced itch (eg, claustrum, insula, and putamen) and was associated with altered cerebral perfusion activity in the midbrain, thalamus, S1, insula, and cerebellum. Thus, butorphanol may be useful in reducing spinal and cortical processing of incoming itch signals, making it an attractive candidate for itch management for AD patients. Formal randomized controlled trials are still needed to evaluate this possibility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




