Acute phase proteins, cytokines and chemokines
Adhesion molecules and ECM components
Factors directly affecting metabolism
α1-acid-glycoprotein
α2-macroglobulin
Adipocyte fatty acid binding protein (aP2)
Chemerin
Collagen I, III, IV, VI
Adiponectin
Chemokine (C-C motif) ligand 5 (CCL-5)
Fibronectin
Adipsin
CRP
Gelsolin
Apelin
Haptoglobin
Intercellular adhesion molecule-1 (ICAM-1)
Apolipoprotein E (apoE)
Interferon (IFN)-β and IFN-γ
Lysyl oxidase
Cholesteryl ester transfer protein
IL-1, 4, 6, 8, 10, 15, 17D, 18
Matrix metalloproteinase (MMP)-1,2,7,9,10,11,14,15
Leptin
IL-1Ra, sIL-1R, IL-1RII, sTNFR
Vascular cell adhesion molecule-1 (VCAM-1)
Lipoprotein lipase
IFN-γ inducible protein 10 (IP-10)
Omentin
Macrophage inhibitory protein-1 alpha (MIP-1α)
Resistin
Macrophage migration inhibitory factor (MIF)
Retinol binding protein 4
MCP-1
Vaspin
Plasminogen activator inhibitor (PAI)-1
Visfatin
Serum amyloid A3
TNF-α
Growth and angiogenic factors
Other factors
Angiopoietin 1 and 2, angiopoietin-like proteins
Acylation stimulating protein (ASP)
Fibroblast growth factors (FGF)
Angiotensinogen
IGF-1
CORS-26
Hepatocyte growth factor (HGF)
Complement-like factors
Nerve growth factor (NGF)
Hepcidin
Stromal derived factor-1 (SDF-1)
Nitric oxide
Transforming growth factor (TGF)-α and β
Prostaglandin E2 (PGE2) and prostacyclin (PGI2)
Tissue factor
Tissue inhibitor of metalloproteinases
Vascular endothelial growth factor (VEGF)
Adipose Tissue and Immune System
In the last decade, AT has been recognized to be an active source of several pro-inflammatory cytokines, chemokines, growth factors and complement proteins [74, 75]. Indeed, it is well known that apart from adipocytes, AT contains numerous mature immune cells including macrophages and lymphocytes [11–14] which are important source of inflammatory cytokines and factors as also adipocytes can be. Monocyte infiltration and differentiation in AT has been shown to correlate with adipocyte hypertrophy, as well as body mass [16] and the expansion of AT in obesity is associated with an increased infiltration with macrophages of the M1 or “classically activated” phenotype from the circulation [76]. These macrophages are usually recruited to sites of tissue damage and have been reported to be in a pro-inflammatory state with increased expression of TNF-α [77]. The cellular mechanisms responsible for this enhanced macrophage recruitment remain largely unknown, but it has been suggested that dysregulated adipokines production and increased adipocyte size might contribute to this phenomenon in a crosstalk between adipocytes and macrophages [78]. More recently, different surveys have shown that AT harbors mast lineage cells that are able to home to organs such as intestine and skin where they fulfill their role. The role of AT as a reservoir of mast cell precursors is interesting according to its strategic location (disseminated all over the body, in the vicinity of visceral organs or skin) and rich vascularization (allowing efficient precursor emigration). In normal physiological states, AT contains only a few mature mast cells whereas their number is increased in obesity [79–81]. Since mast cells produce a large panel of multifunctional molecules including cytokines, growth factors, or enzymes [82, 83] they may contribute to the secretory potential of AT. Expansion of AT, which occurs in obesity, is characterized by chronic low-grade inflammation (persistent and elevated levels of inflammatory factors, including IL-1β, IL-6, and TNF-α) [84]. The consequences of this inflammatory state are also especially dire at epithelial tissues where intraepithelial γδ T lymphocytes normally help maintain barrier function and protect from pathogens [85–88]. However, γδ T cells are sensitive to inflammation and their function becomes impaired by obesity-associated inflammation. AT expansion results in the recruitment of cytotoxic CD8+ T cells and macrophages with increased release of TNF-α, IL-6, and IL-1β as well as depletion of regulatory T cells (Tregs). These events contribute to an increase in systemic inflammation that results in the dysregulation of γδ T cell function and subsequently a decline in barrier homeostasis which may lead to impaired wound healing, increased infections, and susceptibility to inflammatory disease [89]. Moreover, the strict relationship between AT and immune system has always been highlighted by the anatomical close contact between lymph nodes and AT (e.g. lymph nodes are generally surrounded by pericapsular AT). Histological examination of the outer capsule of lymph nodes reveals a fairly thin, loose layer of collagenous material, with numerous very fine lymph vessels that branch from the main vessel and enter the node over almost its entire surface [90]. Such tiny vessels are permeable to large molecules and certain small cells [91] and their arrangement increase the area of vessels passing through the AT immediately surrounding the node, where they may take up lipolytic products released by adjacent adipocytes into the extracellular space. Other sites where lymphoid tissues are in similar intimate contact with adipocytes include the omentum [91, 92] and bone marrow: [93] AT around lymph nodes is a specialized tissue whose function is strictly correlated with lymph node cell populations (e.g. lymph node lymphocytes and tissue dendritic cells (DCs) that acquire their fatty acids from the contiguous adipocytes potentially being influenced by them [94]. Therefore, lymphocytes are often found in close proximity to the adipocytes surrounding the lymph node; consequently, there may be paracrine relationships between the lymphocytes and adipocytes, allowing the exchange of information between the two [17]. However, despite the significative and non negligible presence of immune cells in AT as well as the close contact between lymph nodes and AT, researches into the functional association between AT and the immune system only began in the early 1990s, when adipsin secreted from adipocytes was shown to be identical to complement factor D produced in the immune system [95, 96]. Since then, many more protein secretions and/or cytokine receptors have been described [97]. In most cases, cytokines such as TNF-α were isolated first from the immune system and later found to be secreted by and/or taken up by adipocytes, but others, notably leptin [98], were identified first in AT and later shown to modulate immune function.
Adipokines and Other Mediators: Role in Inflammation
AT is highly dynamic and interactive, playing a central signaling role in the regulation of energy homeostasis, appetite, inflammation, and insulin sensitivity. However in contrast to numerous and excellent publications describing its role in metabolic syndrome and atherosclerosis [99, 100], reviews on the inflammatory role of AT outside the field of metabolism are rare [18, 78, 101]. AT plays a major role in inflammation through the production of a huge class of mediators, the adipokines. Since both adipocytes, macrophages and other cell types present in AT are a non neglectable source of bioactive mediators, they might together perpetuate a vicious cycle of macrophage recruitment and production of pro-inflammatory cytokines, playing a major role in the development and sustainment of inflammatory conditions, especially including skin diseases also due to the anatomical and strict relationship between skin system and AT.
Adiponectin
Adiponectin, also known as Acrp30, AdipoQ, apM1, and GBP28, is a 30-kDa polypeptide highly and specifically expressed in differentiated adipocytes which circulates at high levels in the bloodstream [102]. It is mainly expressed in adipocytes, being higher in subcutaneous than visceral AT [103]. Adiponectin shows anti-inflammatory effects on endothelial cells through the inhibition of TNF-α induced adhesion-molecule expression [104], interferes with the function of macrophages [104, 105], induces the production of important anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist (IL-1RA), by human monocytes, macrophages and DCs, hampers IL-6 production, inhibits the actions of TNF-α and suppresses the production of interferon (IFN)-γ by lipopolysaccharide (LPS) stimulated human macrophages [105, 106]. Moreover, the presence of adiponectin in T-cell proliferation assays results in a decreased ability to evoke an allogeneic T-cell response, and adiponectin also markedly reduces the phagocytic capacity of macrophages as well as TNF-α production by macrophages [106]. Adiponectin is also able to decrease endothelial vascular cell-adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1 and selectin expression [20] and, by decreasing reactive oxygen species (ROS), is an antioxidant [107]. Adiponectin, acting on natural killer (NK) cells, a key component of innate immune system, suppresses the IL-2-enhanced cytotoxic activity of NK cells without affecting their basal cytotoxicity [108] and is also able to inhibit Toll-receptor activation and its consequences [109]. In addition, it increases nitric oxide production in endothelial cells and stimulates angiogenesis. A strong and consistent inverse association between adiponectin and both insulin resistance and inflammatory states has been established [102, 110]. Taken together, these studies suggest that adiponectin is a unique adipocyte-derived hormone with anti-diabetic, anti-inflammatory, and anti-atherogenic effects. Indeed, adiponectin expression is decreased by pro-inflammatory cytokines such as TNF-α and IL-6 [111, 112] and, in obesity, due to the elevated level of these pro-inflammatory cytokines, serum adiponectin is reduced and negatively correlated with Body Mass Index (BMI) [113, 114].
Leptin
Leptin, a 16-kDa nonglycosylated peptide containing 167 amino acids, is mainly produced by adipocytes presenting as an important mediator of immune-mediated diseases and inflammatory processes [115]. Its synthesis is greater in subcutaneous AT rather than visceral AT [116]. Leptin is considered to be a pro-inflammatory cytokine and it has structural similarity to other pro-inflammatory cytokines such as IL-6, IL-12 and granulocyte-CSF [117]. It promotes proliferation and differentiation of hematopoietic cells, alters cytokine production by immune cells, stimulates endothelial cell growth and angiogenesis, and accelerates wound healing [28, 118]. In monocytes and macrophages, leptin increases the production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12 [119]. Leptin is able to stimulate the proliferation of human circulating monocytes in vitro and up-regulates the expression of activation markers such as CD25 (also known as IL-2Rα) and CD71 (the transferrin receptor) on these cells [120]. Furthermore, leptin also activates neutrophils, as assessed by increased expression of CD11b, stimulates neutrophil chemotaxis and the production of ROS by these cells, all of which are very important in innate immune responses and regulation of pathogen colonization of the skin and mucosa. This protein also regulates NK cells differentiation, proliferation, activation and cytotoxicity [121]. Further known direct actions of leptin on immune responses include the promotion of phagocyte function [122], the induction of the synthesis of eicosanoids [123] and nitric oxide [124], the protection of DCs from apoptosis and promotion of their LPS-induced maturation and of a cytokine production profile featuring low levels of IL-10 and high levels of IL-12, TNF-α and costimulatory molecules, which favours the proliferation of allogeneic CD4+ T cells [125]. Other major actions of leptin appear to occur on the level of adaptive immune responses, mainly in T cells regulation. Leptin induces cytokine producing capacity switch towards TNF-α and IL-2 [98, 126], particularly by increasing IFN-γ. Moreover, leptin causes generation, maturation, and survival of T cells, protecting them from apoptosis. It should however be noted that in models of severe inflammation, leptin appears to exert suppressive effects which are contrary to those described above leading to decrease of Th1 type cytokines, increase of Th2 cytokines and reduction in T cells proliferation. Thus leptin effects on the immune system appear to depend not only on the leptin concentrations, but also on the status of the immune system [127]. The complexity of the picture is increased by findings that leptin deficient mice show resistance to certain autoimmune diseases and the susceptibility is recovered by leptin administration [128, 129]. Indeed, leptin may lead to enhancement of autoimmune reactions, in part by reducing Tregs; in addition, its levels are reported to be increased in patients with autoimmune diseases [126]. Moreover, inflammatory cytokines, including TNF-α, and IL-1 are able to induce leptin production [130] with leptin deficiency itself being a known cause of impaired T cell-mediated immunity [131].
Resistin
Resistin, also known as ADSF and FIZZ3, is a 12.5 kDa protein secreted from adipocytes and macrophages that mostly circulates as a high-molecular-weight hexamer, appearing to have many features of an inflammatory cytokine [132]. Greater levels of resistin expression in AT are displayed by monocytes and macrophages versus adipocytes [133]. In humans, resistin appears to play a role in inflammation regulated by pro-inflammatory cytokines, including TNF-α and IL-6 which are also produced by AT. Indeed, resistin stimulates the production by various cell types of inflammatory cytokines such as IL-1, IL-6, IL-12 and TNF-α through a NF-kB-dependent pathway [134–136]. Particularly, even if resistin is minimally expressed in primary adipocytes, these may be target cells for resistin itself: it has been demonstrated that resistin could induce the expression of IL-6, IL-8, and TNF-α by AT [137]. In addition, resistin is also able to up-regulate endothelial cell production of ICAM-1, VCAM-1, endothelin-1 and MCP-1 [138, 139], thus producing a biochemical profile of dysfunctional endothelium. Moreover, resistin mRNA has been also found in human peripheral blood mononuclear cells (PBMCs) and it was reported that pro-inflammatory mediators such as IL-6, TNF-α, IL-1β, IL-12 or LPS can strongly increase the expression of resistin in PBMCs, and that resistin itself is able to stimulate its own production and the secretion of TNF-α, IL-1β, IL-6 and IL-8 in PBMCs as well as IL-12 in macrophages, creating a vicious cycle and suggesting a role in the process of inflammation [134–136].
Visfatin
Visfatin, also known as PBEF, has recently been identified as an adipocytokine that is secreted by adipocytes [140]. However, visfatin is not only produced by AT, but also by endotoxin-challenged neutrophils, in which it prevents apoptosis through a mechanism mediated by caspases 3 and 8 [141]. Circulating visfatin levels are closely correlated with AT accumulation. Visfatin mRNA levels increase in the course of adipocyte differentiation, and its synthesis is regulated by several factors, including glucocorticoids, TNF-α, IL-6 and GH [142]. This molecule binds to and activates the insulin receptor but does not compete with insulin, which indicates that the two proteins bind different sites on the insulin receptor. Visfatin was originally identified more than 10 years ago and since then, it has been linked to several inflammatory disease states [143, 144]. Furthermore, expression of visfatin has been shown to be up-regulated in activated neutrophils and to inhibit their apoptosis [141]. It has been shown that visfatin is able to induce chemotaxis and the production of IL1-β, TNF-α, IL-6 and co-stimulatory molecules by CD14+ monocytes, and to increase their ability to induce alloproliferative responses in lymphocytes [145]. By induction of co-stimulatory molecules such as CD80, CD40 and ICAM-1 visfatin promotes the activation of T cells [146]. Visfatin was also identified in inflammatory cells (lymphocytes) and its levels were reported to be increased in various inflammatory conditions [140]. However, future studies of the cell biology of this natural insulin mimetic and potential inflammation-regulating adipokine should help to define its role in insulin resistance and associated inflammatory disorders.
Chemerin
Chemerin is a 18 kDa chemokine, also known as TIG2 and RARRES2, that was found to be expressed primarily by mature adipocytes and found in ng/mL ranges in human plasma [147]. Various cell types involved in innate and adaptive immunity [plasmacytoid DCs (pDCs), myeloid DCs, macrophages and NK cells] express the orphan G protein-coupled receptor chemokine-like receptor-1 (CMKLR1), and chemerin is now known to function as a chemoattractant that promotes the recruitment of these cells to lymphoid organs and sites of tissue injury acting hence as a pro-inflammatory agent [148–151]. For example, it was found to promote the clustering of the Very Late Antigen (VLA)-4 and VLA-5 integrins at the cell surface, leading to adhesion of macrophages to VCAM-1 and fibronectin [152]. Serum chemerin levels correlate with levels of the pro-inflammatory cytokines TNF-α, IL-6 and CRP [153, 154]. Moreover, chemerin, whose synthesis is increased by TNF-α and Il-1β [155], seems to be able to decrease adiponectin and leptin expression [147]. While initial studies suggested that chemerin might modulate adipogenesis [156, 157] and that functional chemerin receptors were present on both immune cells and AT, little is known of its endocrine or paracrine roles.
Adispin, Apelin, Hepcidin and Vaspin
Adipsin, also known as complement factor D, is a serine protease mainly produced by adipocytes as well as monocytes and macrophages resident in fat [158, 159]. It presents high levels of expression in AT and its circulating concentrations tends to correlate positively with the degree of adiposity, being elevated in obesity and reduced in individuals with total lipoatrophy, AIDS related cachexia and anorexia nervosa [160]. Adipsin mediates the rate-limiting step in the complement activation alternative pathway, underlying the role of AT in immune system biology [158, 159].
Apelin, a recently identified adipokine, which acts thorough the binding to a specific G-protein-coupled receptor named API, present on endothelial cells, vascular smooth myocytes, and cardiomyocytes [161, 162]. It is synthesized in adipocytes and strongly up-regulated by insulin; high plasma apelin levels were found in obese humans [163]. TNF-α may act as a key player in the up-regulation of apelin expression in adipocytes both in obese and lean humans [164]. It has been reported that apelin has a regulatory effect on neoangiogenesis, lymphangiogenesis and fibrogenesis [165, 166].
Hepcidin, first described as a small antimicrobial factor, is a peptide of 25 amino acids which regulates iron homeostasis inhibiting iron absorption by enterocytes, iron release from macrophages, and iron transport across the placenta [167, 168]. Even if it is mainly produced by the liver, AT is able to express hepcidin at both mRNA and protein levels and this expression is reported to be enhanced in obese patients [169]. Levels of hepcidin correlate with levels of CRP and IL-6 [168, 169] and they are reported to be associated with obesity but not liver disease [170]. Indeed, hepcidin expression in AT appears to be stimulated rather by inflammatory stimuli than by iron, particularly through IL-6/STAT3 pathway [171, 172].
Vaspin (visceral adipose-tissue derived serine protease inhibitor), also known as serpinA12, has similarities to adiponectin in that it improves insulin sensitivity. It is a serine protease inhibitor mainly but not exclusively produced by AT (e.g. it can be secreted by skin, stomach, hypothalamus, etc.) which is able to reduce levels of leptin, resistin, and TNF-α; [173, 174] it was also reported to be able to protect endothelial cells from pro-inflammatory cytokines induced inflammation and expression of adhesion molecules [175, 176] showing its multiple potential anti-inflammatory effects.
TNF-α
TNF-α is a cytokine present as either a 26 kDa transmembrane monomer or a 17 kDa soluble molecule. The TNF-α from AT is thought to be primarily produced by macrophages though isolated adipocytes are also known to produce this cytokine [59, 177]. Adipocytes also express both types of TNF-α receptors as membrane bound and soluble forms [178]. TNF-α is a powerful local regulator within AT, acting in both an autocrine and a paracrine manner to influence a range of processes, including apoptosis [97]. Moreover, TNF-α changes the expression of several adipocyte secreted factors, being also the key regulator of several pro-inflammatory compounds; particularly, it enhances the production of IL-6, MCP-1, resistin and visfatin whereas it is able to decrease adiponectin and leptin concentrations [179–181]. TNF-α stimulates adhesion of monocytes to the surface of endothelial cells by enhancing the expression of adhesion molecules (ICAM-1, VCAM-1) being a key cytokine of inflammatory processes [182]. AT expression of TNF-α is increased in obese humans and is positively correlated with adiposity and insulin resistance [178, 183] so that it appears as one of the key links between metabolic disorders and TNF-α mediated skin diseases [e.g. psoriasis and the development of the so-called psoriatic march [184] as well as hidradenitis suppurativa (HS) [185, 186]; indeed, both skin disorders are linked with obesity and metabolic syndrome].
IL-6
Approximately 20–30 % of IL-6 in the circulation is produced by AT [187]. However, IL-6 can be secreted by numerous cell types present in AT with macrophages contributing up to 50 % of AT-derived IL-6 [16] with around 10 % of circulating IL-6 being attributed to synthesis by adipocytes [61]. Within AT, IL-6 and IL-6R are expressed by adipocytes and AT matrix [59]. Like leptin, production of IL-6 by AT increases with increasing adiposity, and circulating IL-6 concentrations are highly correlated with percentage of body fat [188]. Expression and secretion of IL-6 are 2–3 times greater in visceral relative to subcutaneous AT [48, 59]. IL-6 synthesis and secretion by adipocytes is thought to be constitutive but is also stimulated by numerous factors including β-adrenergic agonists [187] and IL-1β [189]. Initial studies also indicated that endothelin-1 may induce adipocyte secretion of IL-6 [190]. Several studies have associated IL-6 polymorphisms with obesity [191] but clear mechanisms have yet to be identified. Circulating IL-6 is the single most important factor controlling the hepatic acute-phase response, the rapid, coordinated physiologic reaction to tissue damage or infection designed to recruit host defense mechanisms, eliminate damaged cells, contain pathogens, and begin tissue repair [57]. Indeed, IL-6 is known to increase the secretion of CRP, a clinically used marker of inflammation, as well as the synthesis of other acute phase response proteins including fibrinogen, serum amyloid-A and α-1 antichymotrypsin [192]. Moreover, IL-6 is also able to inhibit adipogenesis and decrease secretion of anti-inflammatory factors such as adiponectin.
Other Cytokines
Numerous other cytokines are present in AT. For example, IL-15 is secreted from AT, has several functional similarities to IL-2, and it is thought that these two interleukins negatively regulate one another. IL-10 secretion from human AT has also been described [20]. The expression of IL-17D has been showed in adipocytes; [193] it seems that this cytokine is able to stimulate the production of IL-6 and IL-8 from endothelial cells [193]. IL-8 is secreted from both AT explants and cultured adipocytes [194] though others have suggested that IL-8 expression and production is primarily from non-adipocyte cells found in AT [195]. Production and concentrations of IL-8 appear to be AT depot dependent and stimulated by TNF-α [194, 196], differentially regulated in lean and obese men [197] as well as women [198] and influenced by plasma non-esterified fatty acids in overweight men and women [199]. IL-8 production in AT may provide another link between AT and obesity associated diseases. In addition, several reports indicate that circulating levels of IL-18 are elevated in obesity and that they fall following weight loss [200, 201], raising the possibility that AT may be an important direct source of this cytokine in plasma. Indeed, adipocytes and the stromal-vascular fraction of AT are also able to produce IL-18, raising the possibility of cross talk between adipocytes and other cellular components within the tissue [202]. IL-18 acts as a pleiotrophic pro-inflammatory cytokine inducing the expression of chemokines, cytokines, angiogenesis-related, and adhesion molecules such as IL-8, TNF-α, vascular endothelial growth factor (VEGF), and ICAM-1 [203–205]. Even if TNF-α stimulation is able to significantly increase IL-18 production in adipocytes [202] they seem unlikely, however, to contribute significantly to the circulating levels of this cytokine and to the increased levels associated with obesity; therefore, in contrast to other inflammation related factors such as IL-6, TNF-α, MCP-1, and adiponectin, IL-18 cannot be considered a major adipokine.
MCP-1
MCP-1, also referred to as chemokine (C–C motif) ligand (CCL)2, is a chemokine that recruits monocytes to sites of inflammation and has been shown to induce insulin resistance and macrophage infiltration in AT [206]. MCP-1 is expressed and secreted by both adipocytes and stromal-vascular cells [207]. Increased circulating MCP-1 is associated with increased circulating monocytes [208] and MCP-1 expression appears to be highly regulated by TNF-α [209] and, to a lesser extent, by IL-6 and GH [210]. MCP-1 exhibits depot-dependant differences in expression [55] and appears to respond to surgically induced weight loss with a decrease in gene expression [211]. Obesity is associated with increased AT infiltration by macrophages [16, 207] and activated macrophages are able to secrete inflammatory factors, including TNF-α and IL-6. MCP-1 is increased by leptin, obesity, and insulin-resistance-inducing hormones [212].
CRP
The possibility that AT directly contributes to the circulating pool of CRP is suggested by a study which showed that the gene encoding CRP is expressed in AT [213]. However, it is not clear whether CRP expression in adipocytes is significant. Indeed, it seems that AT is not so able to produce significant amounts of CRP [18]. However, IL-6 is highly secreted by AT in obesity and this is the major cytokine regulating the hepatic production of CRP [57, 214]. Thus, AT may be a major player in the raised circulating levels of CRP in obesity, but through the indirect route of adipocyte-derived IL-6. The circulating level of CRP rises with BMI [215, 216], and elevated levels of this inflammatory marker have been associated with both obesity and diabetes, falling with weight loss [217].
As showed above, AT is able to produce plenty of factors so that it could deeply influence the inflammatory response and immune system. Even if it is important to note that these mediators are not all exclusively derived from AT, the factors mainly produced by adipocytes (e.g. adiponectin, leptin and to a less extent visfatin and resistin), can circulate at high concentrations making AT an active and complex actor in inflammation and immunity (Table 14.2).
Adipocytokines | Inflammatory effect | Effects on Innate immunity | Effects on adaptive immunity |
|---|---|---|---|
Adiponectin | Anti-inflammatory | ↓Endothelial adhesion molecules ↓ NF-kB ↓ TNF-α ↓ IL-6 ↓ IFNγ ↑ IL-10 ↑ IL-1RA ↓ Phagocytosis | ↓ B-cell lymphopoiesis ↓ T-cell responses, activation and proliferation ↓ T-cell activation and proliferation |
Leptin | Pro-inflammatory | ↑ TNF-α ↑ IL-6 ↑ IL-12 ↑ Neutrophil activation ↑ ROS ↑ Chemotaxis ↑ NK-cell function | ↑Lymphopoiesis ↑ Thymocyte survival ↑ T-cell proliferation ↑ T-cell activation ↑ TH1 response (IL-2 and IFNγ) ↓ TH2 response (IL-4) |
Resistin | Pro-inflammatory | ↑ TNF-α ↑ IL-1β ↑ IL-6 ↑ IL-12 ↑ NF-kB ↑ Endothelial adhesion molecules | – |
Visfatin | Pro-inflammatory | ↑ IL-6 ↑ IL-8 ↓ Apoptosis of neutrophils | – |
Adipose Tissue in Dermatologic Inflammatory Diseases
As the outermost protective barrier of our body, the skin consists of three layers: epidermis, dermis and a subcutaneous layer which is mainly composed of AT. Given that AT is able to secrete various bioactive proteins, it is not surprising that it may have dynamic functions in skin physiology and pathophysiology. For example, by producing various interleukins, subcutaneous AT may regulate B- and T-lymphocytes in concert with the epidermal keratinocytes, deeply influencing cutaneous inflammation and the development of several skin diseases [1]. Without any doubts, psoriasis represents the dermatologic inflammatory disorder which has been most intensively investigated regarding relationships with increased fat mass and adipokines. However, the literature is also constantly enriched with studies which try to elucidate the role of AT and adipokines in different skin diseases, such as atopic dermatitis (AD), HS, etc., highlighting the growing interest in this topic.
Psoriasis
Psoriasis is a chronic skin inflammatory disease which is now considered a systemic immune mediated disorder. It is well established that obesity is a risk factor for psoriasis [218, 219]. Obesity is one of the most common psoriasis comorbidities. Indeed, obesity prevalence is significantly higher than in general population [218, 220] and it appears to be associated with increased morbidity of psoriasis [221, 222]. Psoriasis is positively correlated with higher BMI, which is also associated with more severe psoriasis and negatively impacts long-term treatment options [223–225]. Moreover, there is increasing evidence that progressive weight loss can produce significant improvements in the severity of psoriasis [226–228] and another direct evidence that obesity may be causal in psoriasis is the fact that bariatric surgery can produce rapid remission from psoriasis [227, 229, 230]. Obesity, with its low-grade systemic inflammation state, and in particular AT, with its immune and endocrine roles, are able to influence the pathogenesis and the development of psoriasis in numerous different ways. For example, the consequences of obesity induced chronic inflammatory state are also especially dire at epithelial tissues with the resulting dysregulation of γδ T cells which may contribute to susceptibility to psoriasis in obese subjects. Indeed, a reduction of a subset of γδ T cells (Vγ9Vδ2) in peripheral blood and their subsequent increase in the epidermis has been documented in psoriasis patients [231]. These cells were able to produce inflammatory cytokines and mediate crosstalk with keratinocytes, suggesting a role for these T cells in the development and the exacerbation of the dermatosis [231]. However, a crucial role in the connection between obesity and psoriasis is indubitably played by the adipokines secreted by AT. Various cells in AT are able to produce TNF-α, as well as other cytokines involved in psoriasis, such as IL-1, IL-6, IL-17 and IFN-γ [20, 66, 97, 232]. The abnormal adipokine levels reported in psoriasis suggest that the systemic inflammation associated with the disease may be linked with AT inflammation, similar to that seen in obesity. Central obesity is associated with greater amounts of inflammatory visceral fat, which is more hypertrophied, contains more macrophage infiltration, has an increased presence of activated T cell populations and expresses a more pro-inflammatory cytokine profile, marked by increased TNF-α, IL-6 and IL-17, and decreased adiponectin [16, 77, 233]. Interestingly, a similar inflammatory state of activated T cells and increased pro-inflammatory cytokines has been described in psoriasis, and is thought to be responsible for the induction of psoriatic plaque formation [234]. All the above mentioned factors, as well as other adipokines such as leptin, are recruited and stimulated in obesity and may have an autocrine and paracrine effect on nearby skin [232, 235]. In particular, leptin levels (both serum and tissue leptin) are enhanced in psoriasis also due to possible functional polymorphism of its gene [236–238] and their concentrations have been also shown to correlate with psoriasis severity [237]. In addition, tissue leptin receptor expression is reported to be significantly higher in patients with severe psoriasis than in patients with mild–moderate psoriasis and controls [237]. Leptin decreases T cells autoregulation, is potentially involved in inflammatory processes stimulating cytokine release and its raised levels may mediate proliferative and antiapoptotic activities in a number of cell types including T cells as well as the increased production of pro-inflammatory cytokines such as IL-6 and TNF-α, some of the major cytokines acting in psoriasis pathogenesis [126, 239, 240]. Moreover, leptin synergistically with IL-1β enhances the production of the antimicrobial peptide human βdefensin-2 (hBD2), whose levels are elevated in psoriasis [241, 242]. In particular, hBD2 seems to be involved in the development of skin inflammation by binding to the CC chemokine receptor 6, consequently inducing chemotaxis of memory T cells or immature DCs and the production of IL-6, CCL20, and CCL5 in keratinocytes [243, 244]. The released hBD-2 may in turn act on keratinocytes, inducing their proliferation and production of pro-inflammatory cytokines/chemokines in an autocrine/paracrine manner, sustaining the dysregulations observed in psoriasis [243]. Thanks to all these proprieties, leptin is able to deeply regulate immune response, being involved in psoriasis pathogenesis, especially its effects on T cells differentiation to Th1 phenotype, induction of pro-inflammatory cytokines by keratinocytes and promotion of keratinocyte proliferation as well as angiogenesis [126, 245–248]. However, there are other several adipokines which are both dysregulated in obesity and psoriasis. For example, resistin leads to up-regulation of inflammatory processes, including TNF-α secretion, is increased in serum of patients with psoriasis, correlating with obesity and increased severity of psoriasis [249, 250]. These evidences support the view that resistin may be involved in the pathogenesis of psoriasis, especially in overweight individuals, possibly by augmenting cytokine expression by the inflammatory infiltrate. On the other hand, levels of adiponectin are lower in psoriatic skin compared with controls (also independently of cardiometabolic risk factors) and in obese psoriatic patients versus normal weight psoriasis subjects [251, 252]. In addition, it has been shown that patients with psoriasis, plasma adiponectin negatively correlated with psoriasis severity and that successful anti-inflammatory treatments may elevate adiponectin levels [253, 254]. All these observations constitute some indirect evidence that the immunological and metabolic alterations associated with obesity may be linked with the pathophysiology of psoriasis. Further data which highlight the complexity of the biologic relationship between AT and psoriasis came from Albanesi et al. who reported elevated serum chemerin levels in psoriatic patients and expression of chemerin in early psoriatic lesions as well as pre-psoriatic skin adjacent to active lesions, in parallel to infiltration by pDCs and neutrophils [255]. However, in chronic plaques low chemerin levels and low pDCs infiltration were found whereas immunoreactive chemerin was present in fibroblasts, mast cells and endothelial cells in early lesions [255, 256]. Taken together all these evidences suggested a role for chemerin in the early phases of psoriasis development. Conversely, the relationship between increased fat mass, psoriasis and adipokines alteration is not always so clear and linear as is highlighted by few papers which show controversial results compared with the above mentioned [250, 257]. In this context the case of an adipokine, such as vaspin, is paradigmatic since it is reported to be elevated in serum of obese subjects [258] whereas serum vaspin is not enhanced in psoriasis patients, being also entirely independent of BMI. Moreover, its expression is decreased in lesional psoriatic skin [259, 260]. However, the literature is full of evidences regarding the strict link between obesity and psoriasis; nevertheless, a causal relationship between them has not been fully established yet. Obesity may occur as a consequence of psoriasis or the obese state may well exacerbate the severity of the disease.
Atopic Dermatitis
AD is a chronic, recurrent, pruritic inflammatory disorder of the skin that has reached epidemic proportions throughout the world, especially in children and adolescents who also showed significantly increased rates of obesity versus previous decades [261–265]. Several studies reported that obesity, particularly when it begins early in life, is associated with increased risk and severity of AD in children whereas other investigations provided evidence of an association between obesity and increased AD also in adult individuals, proposing AD as yet another harmful consequence of obesity [266–270]. To date, the mechanism behind the association of obesity and AD remains unclear. However, few studies indicated that the immunomodulatory effects of obesity might be most profound during the first 2 years of life suggesting a possible effect on the immature immune system. Indeed, obesity is associated with a variety of immune sequelae, including accumulation of macrophages and cytotoxic T cells in AT, production of TNF-α and IL-6, and increased secretion of leptin. This has numerous pro-inflammatory effects on the immune system, including proliferation and activation of circulating monocytes and CD41 and CD81 T cells, polarization of T cells toward a Th1 response, activation of NK cells, promotion of neutrophil chemotaxis and upregulation of numerous cytokines [16, 98, 121, 129, 271–273]. Indeed, the prevalence of AD is reported to be somewhat increased in children with high leptin levels [274]. In addition, obesity is associated with low levels of adiponectin, whose reduced expression has also been detected in AD subjects [274]. Moreover, recently Machura et al. reported that children with AD showed elevated apelin concentrations, apelin/BMI ratio and resistin levels versus healthy children despite similar body weight whereas Suga et al. observed that visfatin levels were increased in both serum and skin lesions of AD patients suggesting that these adipokines may be implicated in AD immunopathogenesis [275, 276]. However, studies on visfatin in AD children are controversial since another survey reported lower serum concentration versus healthy controls [275]. Finally, it is also reported that non obese children with AD show an increased prevalence of fatty liver, a known risk factor for lifestyle-related diseases such as obesity, versus non obese healthy controls [277]. The reasons for this association is not well elucidated but it is speculated that AD subjects may present an enhanced permeability of the small intestine which leads to fatty acid dysregulation which is involved in the pathogenesis of both AD and fatty liver [278, 279].
Hidradenitis Suppurativa
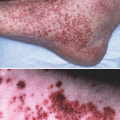
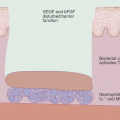
HS is a chronic inflammatory skin disorder characterized by recurrent, painful nodules, abscesses, and draining sinuses, with resultant scarring. Obesity has been associated with HS in up to 75 % of cases and, compared to controls, HS was significantly associated with higher BMI [280–282]. In addition there seems to be a relationship between obesity and the severity of HS so that obesity is considered as a HS severity risk factor [283, 284]. Moreover, weight loss of more than 15 % is reported to be associated with a significant reduction of disease severity [285]. In general, it seems that obesity is able to confer a 1.2-times greater risk for developing HS [282]. Consequently, obesity is considered to participate in the pathophysiology of this skin disorder [280, 281]. A disturbance of the cutaneous innate immune system has been implicated in the key pathogenic steps of HS such as follicular occlusion and chronic inflammation [281]. Moreover, HS has been associated with abnormal expression of antimicrobial proteins, deficiency of IL-20 and IL-22, and elevated TNF-α, IL-1β, and IL-8 levels leading to sustained inflammation and chronic pro-inflammatory state [186, 286–289]. Since AT is a significant source of different pro-inflammatory molecules, which levels are up-regulated when AT is excessively present such as in obesity, it is not unreasonable to hypothesize that AT and its secretory products may participate in the patho-mechanisms of HS and the related cutaneous immune system dysregulations. Indeed, obesity is causally linked to a systemic low-grade inflammatory state with increased levels of pro-inflammatory cytokines in the blood (e.g. IL-1, IL-6, IL-8, TNF-α, CRP) [97, 290, 291] and many of these factors are enhanced and involved also in HS pathogenesis. Furthermore, PBMCs in the obese are in a constant pro-inflammatory mode and demonstrated a significant increase in NF-kB binding and an increase in the transcription of pro-inflammatory genes regulated by NF-kB in these cells [292]. Therefore, even if obesity may not be the primary causative factor in HS, the above mentioned data suggest how it may contribute to HS pathogenesis. However, since a pro-inflammatory state has been also thought to be the driving force for an increase in obesity and metabolic syndrome, to date it is not known if the HS triggers these pathologies or if obesity and metabolic disorders predispose the individual to the development of the inflammatory skin disorder.
Other Skin Diseases
Several links between adipokine dysregulation and skin inflammatory diseases, pathologies which are characterized during their natural course by cutaneous inflammatory phases and/or a more widespread alteration of the immune system are reported in literature. For example, laboratory assessments of patients with systemic lupus erythematosus (SLE) indicated elevated leptin levels which might contribute to systemic inflammation even if to date the clinical and pathogenic significance of this elevation remains unknown [293–296]. However, Yu et al. showed that leptin was able to promote Th17 responses in normal human CD4+ T cells, both in vitro and in vivo, by inducing RORγt transcription. Since Th17 cells play an important role in the development and maintenance of inflammation and autoimmunity, the authors hypothesized the involvement of leptin in autoimmunity and in the connection between metabolism/nutrition and susceptibility to autoimmunity [297]. Several other adipokines are reported to be altered in SLE. Indeed, SLE was independently associated with higher resistin levels and the presence of an association between resistin and inflammation, low complement levels, bone mineral density, and renal function is suggested [298, 299]. In addition, lupus erythematous skin lesions significantly express chemerin (in the endothelium of dermal blood vessels and venules of secondary lymphoid organs) and harbor CMKLR-expressing pDCs, suggesting that chemerin is involved in the recruitment of these cells and other leukocyte populations in this disease [148, 300]. Moreover, visfatin concentrations are reported to be significantly higher in patients with SLE which might reflect inflammation in this systemic disease [293]. However, few other surveys showed controversial data investigating adipokines profile in SLE patients (e.g. lower or unchanged levels of leptin and no significant differences for resistin) [301, 302].
In regard to other disorders, serum leptin concentrations were reported to be significantly increased in systemic sclerosis (SSc) patients versus healthy subjects with similar BMI, however without being significantly correlated or associated with disease duration, clinical activity score, skin score, CRP and antinuclear antibody (ANA) test results [303]. The possible involvement of adipokines in SSc development (where inflammation occurs prior to fibrotic response) is highlighted also by Masui et al. who reported that adiponectin serum levels where significantly lower in patients with diffuse cutaneous SSc (dcSSc) versus those with limited cutaneous SSc and that these levels inversely correlated with the activity of progressive skin sclerosis in dcSSc patients, suggesting that adiponectin serum concentrations may serve as a useful marker to evaluate the activity of progressive skin sclerosis in dcSSc [304]. Consequently, the authors hypothesized that decreased serum levels of an anti-inflammatory factor such as adiponectin may be associated with increased IL-6 levels through modulating the TNF-α action, which promotes the inflammatory process in early dcSSc leading to resultant fibrosis. Indeed, other authors investigating adiponectin, IL-6, IL-2, CRP, ANA and antibodies to extractable nuclear antigens levels in 39 SSc patients speculated that adiponectin could play a protective role in skin related changes during SSc by observing that lower adiponectin serum levels were associated with an advanced stage of skin fibrosis and also because adiponectin is reported to exert anti-fibrotic proprieties [305, 306]. All these findings are reinforced by the study of Arakawa et al. who showed that adiponectin mRNA levels were reduced in skin tissues from patients with dcSSc [307]. Finally, another example could be represented by sarcoidosis. Indeed, very recently Harpsøe et al. conducted a study on 75,008 women who were followed during a median time of 11 years in order to investigate a possible aetiological link between obesity and certain autoimmune diseases [308]. In the sub-group of obese women a high risk was observed for sarcoidosis. Nevertheless its aetiology remains unknown; the disease involves immunological changes similar to those seen in obesity, including TNF-α production [309, 310] and its development may depend on a combination of genetic factors, the triggering antigen itself and immune system status in which the immunologic alterations caused by obesity could play a role [311].
Conclusion
AT has both immune and endocrine roles producing numerous molecules including cytokines, chemokines, growth factors, hormones etc. Therefore, given its proprieties and widespread localization in the human body which is also responsible for the strict and extensive contact between skin and subcutaneous AT, it is indisputable that AT is able to significantly influence the immune responses, the immune skin system and consequently the pathogenesis of several cutaneous inflammatory disorders. We have just shown above the main examples of this interesting relationship trying also to highlight its numerous different molecular and biological basis. Without any doubts, further investigations are needed to deeply investigate AT functions and its ability to regulate skin immune system and cutaneous inflammation.
Questions
- 1.
What cell type does NOT constitute normal adipose tissue?
- A.
Adipocytes and pre-adipocytes
- B.
Vascular cells
- C.
Stromal cells
- D.
Lymphoid/hematopoietic cells
- E.
Neutrophils
- A.
Correct answer: (E) Neutrophils do not normally constitute adipose tissue. This cell type can migrate into adipose tissue during inflammatory responses.
- 2.
How can the adipose tissue act as an endocrine organ?
- A.
Secretion of hormone s such as leptin and IGF-1
- B.
Producing GH
- C.
Producing insulin
- D.
Modulating TSH
- A.
Correct answer (A) Secretion of hormones such as Leptin and IGF-1. Adipose tissue does not produce GH, Insulin or modulate TSH.
- 3.
What is the principal adipokine and its relative functions?
- A.
Insulin, which controls appetite.
- B.
Leptin, which regulates growth, metabolism, and behavior
- C.
Growth hormone, which repairs tissue damage
- D.
Testosterone, which controls sex drive.
- A.
Correct answer: (B) Leptin controls growth, metatolism and behavior. None of the other hormones are derived from adipose tissue, and are thus not considered to be adipokines.
- 4.
What is the role of adipose tissue in a chronic inflammatory skin disease such as psoriasis?
- A.
Metabolize drugs used to treat psoriasis
- B.
Enhances the absorption of lipophilic drugs such as acitretin
- C.
Plays a role in suppressing immune responses
- D.
Cells in adipose tissue produce inflammatory cytokines involved in pathogenesis of psoriasis
- A.
Correct answer: (D) Inflammatory cells that reside in adipose tissue can produce a number of cytokines involved in psoriasis, such as IL-1, IL-6, IL-17 and IFN-γ.
References
1.
Klein J, Permana PA, Owecki M, et al. What are subcutaneous adipocytes really good for? Exp Dermatol. 2007;16:45–70.PubMed
2.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5.PubMed
3.
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8.PubMed
5.
Festa A, D’Agostino Jr R, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obesity. 2001;25:1407–15.
6.
Engström G, Hedblad B, Stavenow L, Lind P, Janzon L, Lindgärde F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes. 2003;52:2097–101.PubMed
7.
Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41:317–25.PubMed
8.
Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–56.PubMed
9.
Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree