The sine qua non to best ensure viability of any autogenous tissues used for breast reconstruction is to maximize the appropriate circulatory pattern to that tissue. This overview of tools used in this regard, all based on the physical principles of the Doppler effect, compares the role today of acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry for perforator identification and flap monitoring. The audible Doppler has recognized limitations, but remains the simplest and most universally available device to assist in this purpose. Laser Doppler flowmetry provides a reasonable system for both intraoperative and post-procedure objective monitoring of the chosen tissue transfer.
Predictable and reproducible success in the transfer of autogenous tissues from any donor site for breast reconstruction must begin in the preoperative planning stage. Milton challenged the dictums of his time in recognizing that the sine qua non for best ensuring viability of any flap depended on its “circulation.” The precise identification of direct or indirect perforators nurturing the assortment of available cutaneous flaps for breast reconstruction for just this reason has assumed paramount importance, as proper flap design must ensure inclusion of that perforator. A giant step taken in that direction was by Taylor and colleagues, who first used the still ubiquitous acoustic Doppler probe to identify “dominant” skin perforators, and then planned safe flaps of “unusual dimensions and directions,” along an axis connecting those perforators. The Doppler effect so evoked remains tacitly understood from basic principles of physics. Named after the Austrian physicist Christian Doppler, who actually identified the concept in 1842, this phenomenon is the change in frequency of a wave for an observer moving relative to the source of the wave. This key principle has become ingrained in plastic surgery routines, and has since been expanded to include other evolving technologies such as color duplex ultrasound and laser Doppler flowmetry.
Acoustic Doppler sonography
The typical acoustic Doppler probe is virtually universally available in every operating room and at every nursing station ( Fig. 1 ). This compact device is highly portable, simple to use, and usually easy to interpret.
Acoustic Doppler sonography emits sound waves that reflect on a moving object relative to a fixed observer, and has a distinct phase shift according to the Doppler effect. In flaps, the red blood cells are basically the only moving objects that scatter the incident source. The frequency of the latter will be shifted by an amount proportional to the number and velocity of the moving red blood cells in the specific target region, which predominantly represents intravascular blood flow. This results in the familiar and characteristic audible pitch that we differentiate between arteries and veins.
To increase the specificity for locating a skin perforator, which is necessary to ensure flap boundaries will be designed to include that perforator, Mun and Jeon have suggested a “perforator compression test.” Because perforators are thinner walled and more superficial than their source vessel, and their course is approximately perpendicular to the skin surface, “true” perforators will be more easily compressed by an external force applied perpendicular to the skin surface. Thus, after finding a potential perforator with the audible Doppler probe, increased downward pressure will reduce and almost obliterate the original sound. Gradual release of the pressure will result in increasing loudness. Deeper or source vessels should have little change in sound intensity during such maneuvers.
As with any modality, there are limitations with acoustic Doppler sonography. The sensitivity may actually be too high (false positives), as often diminutive vessels inadequate to sustain a cutaneous flap can be found. The specificity may be too low (false negatives), as vessels can be overlooked or missed because of background noise from larger vessels in the vicinity. This latter characteristic also diminishes the capability of this device to allow accurate and consistent postoperative monitoring.
From a pragmatic standpoint, acoustic Doppler sonography has a short learning curve and will always be used by many, because it is readily available, as the sole technique for preoperative perforator identification, or to corroborate the conclusions gleaned from other diagnostic modalities ( Fig. 2 ). Because the probe can be sterilized, this is still the most practical means for intraoperative verification of perforators, as well as assessment of the location of recipient vessels.
Color duplex ultrasound
If a color spectrum is added to the moving component of conventional high-resolution, B-mode, gray-scale ultrasound, this combination of techniques is color duplex imaging. The actual color observed on the monitor depends on the direction of flow in relation to the transducer used to perform the study. This can arbitrarily be assigned the color red or blue, with red usually chosen for the arteries and blue for the veins to simplify data interpretation, once each vessel type is identified correctly using the audible format included with most commercial machines.
Higher frequency transducers now permit scanning to superficial depths just below the skin level, with sensitivity to detect vessels with a diameter as small as 0.2 mm. This so-called “power Doppler imaging” is particularly relevant for investigations of perforators within the microcirculation, and was first used in plastic surgery for mapping of transverse rectus abdominis musculocutaneous (TRAM) flap perforators, usually then marked by coordinates centered about the umbilicus before breast reconstruction. Fasciocutaneous perforators to deep inferior epigastric artery perforator (DIEAP) flaps, as well as other potential donor sites, have also been similarly localized.
Depending on the orientation of the color duplex imaging transducer, the vessel orifice can be calibrated and peak systolic flow velocity determined from concomitant pulse-volume recordings; the take-off of perforators from the source vessel identified ( Fig. 3 ); and the perforator intramuscular, subfascial, or epifascial course documented.
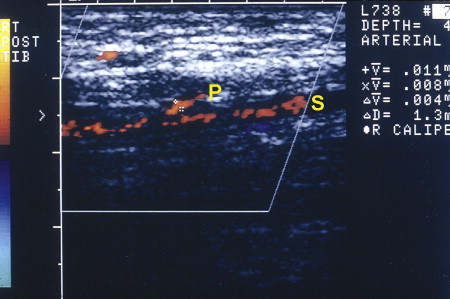
Sensitivity in identifying perforators with color duplex imaging is high, but specificity is low, as only a small region can be examined at any time. This equipment has limited availability, and usually a technician must be present to obtain consistent observations. Postoperative monitoring of buried DIEAP free flaps has been reported, but the apparatus is just too awkward for routine continuous bedside or intraoperative monitoring ( Fig. 4 ).
Color duplex ultrasound
If a color spectrum is added to the moving component of conventional high-resolution, B-mode, gray-scale ultrasound, this combination of techniques is color duplex imaging. The actual color observed on the monitor depends on the direction of flow in relation to the transducer used to perform the study. This can arbitrarily be assigned the color red or blue, with red usually chosen for the arteries and blue for the veins to simplify data interpretation, once each vessel type is identified correctly using the audible format included with most commercial machines.
Higher frequency transducers now permit scanning to superficial depths just below the skin level, with sensitivity to detect vessels with a diameter as small as 0.2 mm. This so-called “power Doppler imaging” is particularly relevant for investigations of perforators within the microcirculation, and was first used in plastic surgery for mapping of transverse rectus abdominis musculocutaneous (TRAM) flap perforators, usually then marked by coordinates centered about the umbilicus before breast reconstruction. Fasciocutaneous perforators to deep inferior epigastric artery perforator (DIEAP) flaps, as well as other potential donor sites, have also been similarly localized.
Depending on the orientation of the color duplex imaging transducer, the vessel orifice can be calibrated and peak systolic flow velocity determined from concomitant pulse-volume recordings; the take-off of perforators from the source vessel identified ( Fig. 3 ); and the perforator intramuscular, subfascial, or epifascial course documented.
Sensitivity in identifying perforators with color duplex imaging is high, but specificity is low, as only a small region can be examined at any time. This equipment has limited availability, and usually a technician must be present to obtain consistent observations. Postoperative monitoring of buried DIEAP free flaps has been reported, but the apparatus is just too awkward for routine continuous bedside or intraoperative monitoring ( Fig. 4 ).
Laser Doppler flowmetry
We have never attempted use of a scanning laser Doppler for preoperative regional perforator identification, but have used surface laser Doppler flowmetry (LDF) for more than 20 years for both the intraoperative and postoperative monitoring of free flaps and sometimes pedicled flaps, following introduction of the concept by investigators from the Duke group. More often than not, potential complications from pedicle kinking, an excessively tight closure, or anastomotic compromise are made apparent in the operating room even before clinical signs of flap embarrassment are obvious. This noninvasive system provides an accurate, objective display that is an almost ideal monitoring system ( Fig. 5 ).
Laser Doppler flowmetry relies on tissue illumination by a coherent laser light, which has an emission wavelength of 780 nm from a 5-mW diode laser, as the reflecting source transmitted via a fiberoptic cable. Although not visible to the naked eye, the Doppler shift of this reflected laser light can be calibrated via a computer within the monitor as a linear function of the average velocity of moving cells within the given flap. Detection of blood flow and flow velocity up to an 8-mm depth is possible. Real-time measurements of tissue perfusion are averaged over a predetermined time frame, and displayed either as a trend line ( Fig. 6 ) or a number (see Fig. 5 ) that nursing personnel or even family members can watch for a significant variation, and does not require constant surveillance by a member of the surgical team.
Although each unit of measurement recorded corresponds to flow in mL/min/100 mg tissue, absolute flow is not measured directly by this device, but rather provides a relative estimate that varies by patient and donor tissue. Falsely elevated or positive flow values have been observed even under “no flow” conditions, and can be caused by tissue vibrations, movement of the patient, or other artifacts. Loss of arterial inflow typically results in an immediate and precipitous drop of flow values toward zero (see Fig. 6 ). To provide a safety factor, we arbitrarily like to be notified if the display value drops below 1.0, which then mandates immediate clinical evaluation of the flap on our part. Venous obstruction is less obvious. A spiraling downward trend over time is consistent with an impediment of venous outflow (see Fig. 6 ). This paradox of persistent positive flow values, albeit progressively smaller, can be explained, as some flow will continue into the flap up until the capacitance of the system is maximized.
Intraoperative laser Doppler flowmetry has also allowed us to compare relative perfusion to zones of the abdomen ( Fig. 7 ). Poorly perfused regions should be discarded. Comparison of the relative perfusion from individual perforators, evaluated after alternating clamping, aids in deciding which perforators and their source vessels should be retained with the flap, which is especially valuable in deciding whether a superficial inferior epigastric artery flap is feasible in lieu of a deep inferior epigastric artery perforator flap. Probably the most critical time for assessing flap perfusion after completion of microanastomosis is during insetting. LDF surveillance during this period (see Fig. 7 ) helps to determine any pedicle compromise from kinking, stretching, or excessive pressure.