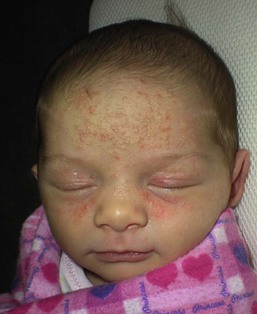
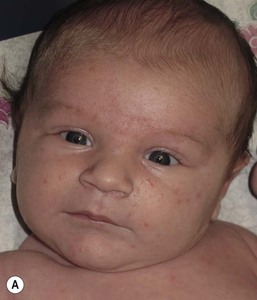
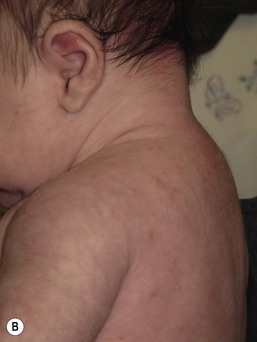
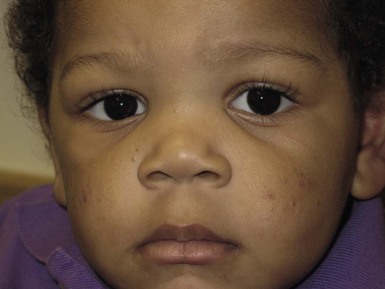
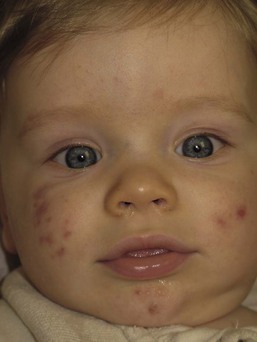
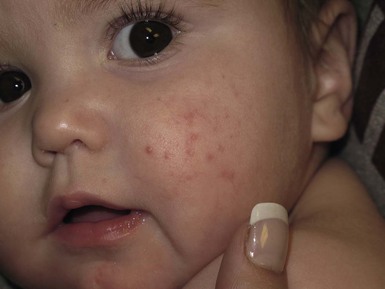
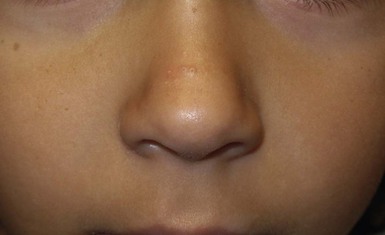
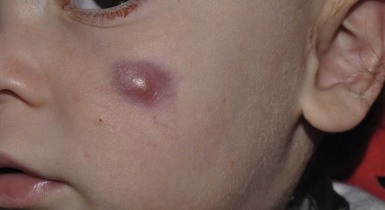
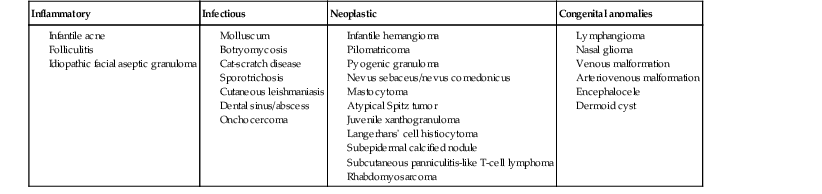
Andrea L. Zaenglein Neonatal acne (acne neonatorum) is a very common newborn eruption, occurring in up to 20% of healthy babies. Lesions are not present at birth but typically appear within the first 2–3 weeks of life and generally improve by about 4 months of age. Small inflammatory red-pink papules and pustules predominate, occurring symmetrically on the cheeks (Fig. 25.1). The forehead, chin, eyelids, scalp, neck, back and chest can be affected as well (Fig. 25.2). Open comedones and nodules are rarely evident, and if present, suggest the possibility of true acne vulgaris, which should be treated according to infantile acne recommendations (see below). The diagnosis of neonatal acne is usually made clinically. Giemsa staining of pustule contents reveals neutrophils and variable yeast spores. The term ‘neonatal cephalic pustulosis’ (NCP) was proposed to help differentiate this common neonatal eruption from true acne.1,2 In contrast to the androgen-driven infantile acne vulgaris, the pathogenesis of neonatal acne is unclear. Increased sebum and the presence of yeast flora have been implicated as possible triggers. It is known that newborn sebum excretion rates correlate with maternal sebum excretion rates perinatally and these levels subsequently decrease over the next several weeks.3 This suggests that both mother and baby are subject to the same hormonal stimulus following birth. Along with these changes, the baby begins to undergo colonization of normal flora. Malassezia furfur, M. sympodialis and other species frequently colonize neonatal skin, with prevalence rates of 35–50% at 1 week of age and increasing with age, peaking at around 90% by 3–6 months of age.4 However, Malassezia species are not uniformly cultured from the pustules of neonatal acne nor does the degree of colonization appear to correlate with severity of the clinical findings.5 Hence, the role of yeast in the pathogenesis of neonatal acne remains controversial. The differential diagnosis of acneiform lesions in the neonatal and infantile period is broad (Box 25.1). More florid cases of neonatal acne are sometimes indistinguishable from seborrheic dermatitis, very early onset atopic dermatitis, miliaria rubra and in rare instances, congenital candidiasis. In atypical cases, serial follow-up and close scrutiny for lesions on other body sites is recommended. The condition is self-limited and treatment is rarely required. However, in pronounced cases, topical imidazoles, such as clotrimazole or ketoconazole cream, or a low potency topical corticosteroid, such as hydrocortisone 1% cream, may be helpful in accelerating resolution. In contrast to neonatal ‘acne’ – a very common condition – infantile acne vulgaris is relatively uncommon. The age of onset is usually between 6 and 12 months, with most cases resolving by 1–3 years.6 Boys are more commonly affected than girls.6–8 Clinically, it is characterized by classic acne lesions occurring primarily on the cheeks. An admixture of open and closed comedones, alone or with inflammatory papules, pustules and small nodules are seen (Figs. 25.3, 25.4). Severe forms with deep, suppurative nodules have been reported.9 The pathogenesis of infantile acne centers on a physiologic increased production of adrenal and gonadal androgens intrinsic to this stage of development. During the first 6–12 months of life, infant boys have elevated levels of luteinizing hormone with a resultant increased production of testosterone.10 In addition, the infantile adrenal gland, in both boys and girls, is still immature with an enlarged zona reticularis that produces elevated levels of dehydroepiandrosterone (DHEA). DHEA is subsequently converted by the sebaceous gland into more potent, acne-promoting androgens, like dihydrotestosterone.11 By 12 months, adrenal androgen levels typically decrease, remaining low until the onset of adrenarche. Testicular androgen production is also minimal throughout childhood.12 It is important to note that the majority of affected babies do not have an overt pathologic cause of hyperandrogenism and laboratory workup is usually unnecessary.6 However, a complete hormonal evaluation is indicated in any infant with acne that is unusually severe, persistent, or if the child has other signs of hyperandrogenism or precocious puberty, such as breast development, testicular hypertrophy, penile or clitoral enlargement, or the presence of axillary or pubic hair. Causes of infantile hyperandrogenism are listed in Box 25.2. The role of genetics in infantile acne is undoubtedly strong with 25–50% of patients reporting a positive family history of severe adolescent acne and 25% noting a sibling with infantile acne.6,7 Most cases of infantile acne resolve by 2–3 years of age, though in some cases, it can last up to age 5 years.13 It is important to treat even mild cases of infantile acne because scarring is fairly common in this age group. Up to 50% of infants with acne develop resultant pitted scars on their cheeks (Fig. 25.5).7 Treatment with a mild retinoid, such as adapalene 1% gel or tretinoin 0.025% cream, may be used daily with a benzoyl peroxide 2.5% cream. Certain formulations such as washes and alcohol based gels should be avoided in infants due to risk of irritating the eyes and skin. For more severe inflammatory lesions, oral erythromycin, azithromycin or trimethoprim-sulfamethoxazole (in infants 2 months and older) can be used. For the very rare cases of severe nodular acne, use of oral isotretinoin is effective.14 As with other forms of acne, treatment for several months is often needed, with gradual tapering of medications as the condition improves. Acne and acne-like lesions have been reported from the use of several medications. Most notably, high dose systemic corticosteroids can cause an acneiform eruption in childhood. The lesions are characteristically monomorphous pink-red papules and pustules erupting on the face, chest, shoulders and upper back. Chloracne, most commonly reported due to industrial or incidental exposure to the toxic hydrocarbon, dioxin, can also cause acne in infants and children.15 Open comedones and small nodules predominate, often involving the malar region and ears, extending to involve the nape of the neck. The central face is spared. Other potential causes of medication-induced acne are listed in Box 25.1. Apert syndrome, or acrocephalosyndactyly, is characterized by craniosynostoses, midface hypoplasia and symmetric syndactyly of the bones of the hands and feet. Defects in skin, skeleton, brain, and visceral organs are commonly associated. Most cases are sporadic, but autosomal dominant and mosaic forms are reported. Apert syndrome is caused by a missense mutation in the fibroblast growth factor receptor-2 (FGFR2) gene that encodes the FGFR2 protein. Interestingly, somatic mutations in the FGFR2 gene have been found in several cases of mosaic nevus comedonicus (acneiform unilateral nevus).16 This growth factor protein is vital for numerous cell processes including cell division, growth regulation and maturation, formation of blood vessels, wound healing, embryonic development and malignancy. Patients with Apert syndrome develop pronounced seborrhea with atypical acne lesions, involving the forearms, buttocks and thighs early in puberty, but to date this has not been described in early infancy.17 The acneiform lesions are notably resistant to standard acne treatments and isotretinoin is often required to control the process.18 More than 80% of patients with autosomal dominant hyper-IgE syndrome (AD-HIES, Job syndrome) report a neonatal papulopustular eruption, most often affecting the face and scalp.19–21 Approximately 24% of babies with autosomal recessive hyper-IgE syndrome (AR-HIES) also have a similar indistinguishable eruption.22 The rash usually appears before 2 months of age, with the majority of cases occurring at or within the first 2 weeks after birth. The predominant lesions are crusted papules and small pustules that will wax and wane in severity. Pruritus is a common feature. The rash is associated with peripheral eosinophilia, elevated IgE levels and variable leukocytosis.19 By age 18 months, most affected infants also develop a generalized xerotic, papular and follicular eczematous dermatitis concentrated on the upper body. A classic atopic dermatitis pattern is seen more frequently in AR-HIES (see Chapter 15).21 The eczematous rash may be secondarily impetiginized by Staphylococcus aureus and recurrent abscesses are typical. Over time, a pattern of recurrent infections begins to develop. Affected children develop recurrent bacterial pneumonia, commonly caused by S. aureus, Streptococcus pneumoniae or Haemophilus influenzae. Opportunistic organisms, such as Pneumocystis jiroveci and secondary fungal infections can also occur, leading to significant morbidity.23 Chronic candidal infections of the oral mucosa, nails, and gastrointestinal tract are also described. Recurrent and resistant cutaneous viral infections, including herpes simplex virus, varicella zoster virus, human papillomavirus, and molluscum contagiosum, characterize AR-HIES and are uncommon features of the autosomal dominant form of the disease.21 Additionally, cutaneous malignancy, developing early in adulthood is seen almost exclusively in AR-HIES.21 Later in childhood, other defining features of AD-HIES become evident. These include a characteristic facies (defined by a coarse texture to the facial skin, a broad nasal bridge and a bulky nasal tip), retention of primary teeth, hyperextensibility of the joints, tortuous medium-sized blood vessels, osteopenia and bone fractures. Histopathologic evaluation of the facial skin eruption is nonspecific but may show eosinophilic spongiotic dermatitis or eosinophilic folliculitis. Laboratory investigations reveal a nonspecific leukocytosis with eosinophilia. Despite the name ‘hyper-IgE syndrome’, serum IgE levels in infancy may be only moderately elevated, indistinguishable from elevations caused by atopy. Diagnosis can be suspected because of the clinical features and confirmed with genetic testing. The differential diagnosis of the early papulopustular eruption includes common neonatal eruptions, such as neonatal acne and miliaria. As the other features of HIES develop, the differential diagnosis expands to include eczematous rashes in association with recurrent infections, including severe atopic dermatitis, tyrosine kinase 2 deficiency (hyper-IgE syndrome with atypical mycobacteriosis), Wiskott–Aldrich syndrome, Netherton syndrome, Omenn syndrome and other causes of neonatal erythroderma (see Chapter 18). The autosomal dominant form of hyper-IgE syndrome is due to mutations in the STAT3 gene, with the majority of cases resulting from de novo mutations in the gene. The autosomal recessive form is due to mutations in the DOCK8 gene. Management of hyper-IgE syndrome is aimed at prevention and directed treatment of infections as well as typical skin therapy for atopic dermatitis. Treatment with topical corticosteroids, antifungal creams and oral antibiotics are variably effective. The transverse nasal crease is a horizontal anatomical demarcation line between the alar cartilage and the triangular cartilage at the lower-third of the nose. Transverse nasal crease can be familial and seen more commonly in atopic patients associated with an ‘allergic salute’.24 Within this developmental fault line, milia, cysts and comedones can form (Fig. 25.6).25 When lesions rupture or are traumatized, inflammatory papules result. The histology of these lesions reveal foreign body granulomatous inflammation.26 This entity is primarily seen in school-age children (4–12 years of age) prior to the onset of puberty, but may be seen earlier, with resolution occurring within months to years.26 Treatment of open comedones consists of surgical expression as needed. Topical retinoids and benzoyl peroxide have been used with variable results. In childhood flexural comedones, noninflamed, open comedones are noted bilaterally or unilaterally in the axilla.27 The groin, neck and antecubital fossa are less commonly involved. The lesions appear in early childhood with one congenital case described. Males and females are equally affected.27 Several of the reported patients had associated molluscum contagiosum infection.27 The lesions do not follow any segmental or Blaschkoid pattern as seen with nevus comedonicus.28 Some authors suggest that childhood flexural comedones could be related to hidradenitis suppurativa.27 Idiopathic facial aseptic granuloma (IFAG; also known as ‘pyodermite froide du visage’) is characterized by one or more persistent, asymptomatic, inflammatory nodules most often located on the cheek. Lesions are typically red to purple and soft to slightly rubbery. The lesion size varies, with a mean diameter of 10 mm (range 3–25 mm) (Fig. 25.7). Most patients have only a solitary lesion, however some have two or three. While most involve the mid-cheek, other sites including the lateral cheek infraorbital skin, or eyelids can be involved.29 While the etiology is unknown, the association with concomitant eyelid chalazia has led some authors to speculate whether this entity is a variant of granulomatous rosacea.30,31,32 Histopathologic evaluation reveals chronic granulomatous inflammation comprised of lymphocytes, histiocytes, neutrophils and many foreign body type giant cells. No shadow cells, cyst walls or calcium deposition should be evident. Cultures for bacteria, fungus and mycobacteria are negative, although a few reported cases were deemed secondarily positive to Staphylococcus aureus, Streptococcus species and Enterococcus faecalis.29 Ultrasonography shows a well-demarcated, hypoechoic dermal nodule without calcification. The differential diagnosis includes infantile acne, folliculitis, atypical Spitz tumor, resolving infantile hemangioma, pilomatricoma, and pyogenic granuloma. Infectious etiologies should also be considered, including botryomycosis, cat-scratch disease, sporotrichosis and cutaneous leishmaniasis. Table 25.1 details an expanded differential diagnosis of nodular lesions on the face of a young child. Treatment of IFAG is often unnecessary as the lesions can resolve spontaneously, though this may take many months. Macrolide antibiotics (azithromycin, erythromycin and clarithromycin) have been used for 2 weeks to 2 months with variable improvement. Topical metronidazole cream has also been used. In persistent cases, incision and drainage or surgical excision can be performed.29 Childhood rosacea is thought to be rare in young children; however, several authors argue that it is more common than generally appreciated because other relatively common childhood conditions such as perioral dermatitis (as well as IFAG) are actually variants of rosacea.30–33 Childhood rosacea is observed more commonly in females with an average age of 4.2 years.33 It has been reported in infants as young as 1 year of age.33 A family history of rosacea is reported in 15% of patients with ocular rosacea.34 Four subtypes are described: papulopustular, telangiectatic, granulomatous and ocular. Children diagnosed with rosacea typically present with a combination of ocular and cutaneous symptoms similar to the findings in adult rosacea. The papulopustular variant is most common with small, erythematous inflamed papules and pustules noted in a malar distribution (see Fig. 25.9). Telangiectasia and macular erythema with or without flushing are common. Ocular manifestations include blepharitis with meibomian gland inflammation and recurrent chalazia, hyperemia, photophobia, episcleritis, or keratoconjunctivitis, and rarely corneal ulceration.33,35 The presence of childhood chalazion (stye) is associated with a higher prevalence of rosacea in adulthood.36 Childhood rosacea is a clinical diagnosis and biopsy is rarely indicated. The differential diagnosis is that of other infantile papulopustular eruptions. Notably, while Demodex mites can be associated with adult rosacea, their presence has not been documented in the childhood form.33 Treatment of rosacea in infants consists of topical metronidazole, azelaic acid, or niacinamide. In refractory or extensive cases, oral erythromycin or azithromycin may also be employed. For ocular symptoms, systemic erythromycin or azithromycin is often used in conjunction with topical ocular erythromycin or metronidazole ophthalmic gel, ocular steroid preparations and diligent eyelid hygiene.33,34
Acneiform and Sweat Gland Disorders
Acneiform disorders
Neonatal acne (neonatal cephalic pustulosis)
Infantile acne vulgaris
Drug-induced acne
Acne associated with apert syndrome
Hyper-IgE syndrome
Pseudoacne of the nasal crease
Childhood flexural comedones
Idiopathic facial aseptic granuloma
Childhood rosacea
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Acneiform and Sweat Gland Disorders
25