Fig. 23.1
Sebaceous lipogenesis is dependent on the fatty acids available. While unsaturated ω3 and ω6 fatty acids are essential, ω9 can be synthetized by the sebaceous glands. The saturated/unsaturated fatty acid ratio defines inflammatory triggering and can initiate comedogenesis
On the other hand, fatty acids exhibit strong antimicrobial activity. The sebaceous ω9-fatty acids sapienate (C16:1δ6), palmitate (C16:0), and oleate (C18:1) are very effective against Staphylococcus aureus (Chen et al. 2011; Georgel et al. 2005; Wille and Kydonieus 2003; Drake et al. 2008). Moreover, dysfunction of the upstream lipidogenic enzymes SCD and FADS-2 is associated with skin infection and inflammation (Georgel et al. 2005; Seltmann et al. 2000). Lipids at the skin surface, mostly secreted from the sebaceous glands (90 %) and transported through the follicular canal, are part of the symbiotic and innate immunity of the skin and contribute to the antimicrobial skin barrier.
PPAR and Acne
Certain lipid mediators, which are able to interfere with sebocyte differentiation and lipogenesis, have been shown to activate and/or be ligands of PPAR (Makrantonaki et al. 2011; Ottaviani et al. 2006; Alestas et al. 2006; Chen et al. 2003; Zhang et al. 2006). Importantly, lipid peroxidation products are also capable of inducing PPAR activation and production of proinflammatory cytokines. In particular, PPARα seems to be related to β-oxidation of fatty acids and lipid catabolism, whereas PPARγ activation has been linked to lipogenesis (Ferré 2004). Eicosanoid metabolites originated from the arachidonic acid cascade, namely leukotriene B4 and 15-HETE, have been shown to be ligands of PPARα and PPARγ, respectively (reviewed in Zouboulis et al. 2005; Alestas et al. 2006). Interestingly, the enzymes involved in their formation, including 5-LOX, have been implicated in inflammatory skin diseases characterized by keratinocyte hyperproliferation (Ottaviani et al. 2006) and have been found to be expressed at higher extent in acne-involved skin in comparison to the skin of healthy subjects (Alestas et al. 2006). Activation of 5-LOX results, among other effects, in induced IL-6 and IL-8 expression in human sebocytes, whereas enhanced expression of IL-6 and IL-8 has also been found in acne-affected skin (Alestas et al. 2006). Systemic treatment of acne patients with the 5-LOX inhibitor Zileuton reduces the inflammatory lesion count and the synthesis of sebum lipids, in particular, of those with proinflammatory potential (Zouboulis 2009) through an inflammation-preventive mechanism (Zouboulis et al. 2010). 5-LOX inhibitors may also downregulate the inflammatory activity of lymphocytes and macrophages resulting in cumulative beneficial effects (Jeremy et al. 2003).
Prostaglandins are further proinflammatory mediators thought to be involved in acne lesion development (Zhang et al. 2006). Mice with increased cyclooxygenase-2 (COX-2) expression and prostaglandins E2 levels showed sebaceous gland hyperplasia and enhanced sebum production (Neufang et al. 2001) suggesting an important role for COX-2 signaling pathway in sebocyte biology. Expression and activation of COX-2 has been shown in in vitro models to be PPARγ mediated. General oxidative stressors, including lipid oxidizing agents, activate PPARγ and induce lipogenesis in sebocytes (Trivedi et al. 2006a, b; Zhang et al. 2006; Ottaviani et al. 2010). All these findings allow the hypothesis that sebocyte proliferation and/or lipogenesis as well as inflammatory reaction, may be regulated by PPARγ-mediated pathways.
Neuropeptides
Corticotropin-releasing hormone (CRH), the most proximal element of the hypophysis-pituitary-adrenal axis, acts as a central coordinator for neuroendocrine and behavioral responses to stress. CRH, CRH-binding protein, CRH-receptor 1, and CRH-receptor 2 are expressed in SZ95 sebocytes at mRNA and protein level, whereas CRH-receptor 1 is the predominant type (Zouboulis et al. 2002). In addition, CRH significantly upregulates mRNA levels of 3β-hydroxysteroid dehydrogenase/Δ5−4 isomerase and induces sebaceous lipogenesis and IL-6 and IL-8 synthesis (Zouboulis et al. 2002; Krause et al. 2007). In acne-involved skin, the complete CRH system is abundant especially in the sebaceous glands, possibly activating lipid pathways, which affect immune and inflammatory processes leading to the development and stress-induced exacerbation of acne (Ganceviciene et al. 2009).
Diet
Evidence suggests that diet may influence acne (Rasmussen 1997; Pappas 2009b; Liakou et al. 2013; Smith et al. 2007), whereas it is also an important source of substrate for the synthesis of sebaceous lipids (Rasmussen 1997). This notion is supported also by the observation that sebum contains essential fatty acids, such as linoleate and oleate. On the other hand, extreme caloric restriction dramatically decreases the sebum excretion rate and these changes can be reversed when a normal diet is resumed (Pochi et al. 1970; Downing et al. 1972). Other studies have demonstrated that increased consumption of dietary fat or carbohydrate increases sebum production and modifications to the type of carbohydrate can also alter sebum composition (Macdonald 1964). Typical western diet, comprised of milk and hyperglycaemic foods, may have potentiating effects on serum insulin and insulin-like growth factor-1 levels, thereby promoting the development of acne (Melnik and Schmitz 2009). In contrast, a low-glycemic-load diet for 12 weeks in acne patients reduced parallelly the acne lesion count and increased the C16:0/C16:1 fatty acid ratio (Smith et al. 2007, 2008) suggesting an increased enzymatic desaturation of fatty acids in the sebaceous glands of patients with acne (Fig. 23.2).
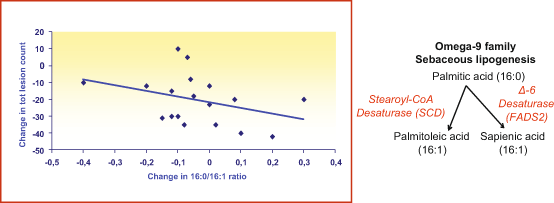
Fig. 23.2
Skin surface lipid composition under a 12-week acne diet. Decrease in the enzymatic desaturation of fatty acids correlates with the clinical improvement in acne
The nutritional cell status is primarily sensed by the forkhead box transcription factor O1 (FoxO1) and the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1) (Wang et al. 2011). FoxO1 attenuates androgen signaling, interacts with regulatory proteins important for sebaceous lipogenesis, regulates the activity of innate and adaptive immunity, antagonizes oxidative stress, and most importantly functions as a rheostat of mTORC1, the master regulator of cell growth, proliferation, and metabolic homoeostasis. Thus, FoxO1 links nutrient availability to mTORC1-driven processes in the skin: increased protein and lipid synthesis, cell proliferation, cell differentiation including hyperproliferation of acroinfundibular keratinocytes, sebaceous gland hyperplasia, and increased sebaceous lipogenesis (Melnik and Zouboulis 2013). Deeper insights into the molecular interplay of FoxO1/mTORC1-mediated nutrient signaling are thus of critical importance to understand the impact of western diet on the promotion of epidemic acne.
Conclusions
Increased sebum excretion, alteration of lipid composition and the oxidant/antioxidant ratio of the skin surface lipids are major concurrent events associated with the development of acne (Zouboulis 2004a; Table 23.1). Current evidence indicates that sebum composition (lipid quality), and not quantity, plays a central role in the development of acne. This concept is supported by the mode of action of new antiacne compounds, such as the 5-LOX inhibitor Zileuton, which reduces acne lesions by inhibiting proinflammatory lipids (Zouboulis 2009; Zouboulis et al. 2010), and the current evidence of the effect of diet on acne (Melnik and Schmitz 2009). Moreover, old data on in vivo and in vitro modulation of sebaceous lipid composition by isotretinoin, the most potent antiacne drug, can be approached from this new perspective (Stewart et al. 1984; Strauss et al. 1987; Melnik et al. 1988; Zouboulis et al. 1991).
Table 23.1
Sebaceous gland functions, which are possibly involved in the development of acne
Production of sebum (Zouboulis et al. 2003) |
Regulation of local androgen synthesis (Fritsch et al. 2001) |
Interaction with neuropeptides (Zouboulis et al. 2002) |
Synthesis of specific lipids with antimicrobial activity (Wille and Kydonieus 2003) |
References
Downing DT, Strauss JS, Pochi PE. Changes in skin surface lipid composition induced by severe caloric restriction in man. Am J Clin Nutr. 1972;25:365–7.PubMed
Downing DT, Stewart ME, Wertz PW, Strauss JS. Essential fatty acids and acne. J Am Acad Dermatol. 1986;14:221–5.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






