ACCESS- FOCUS ON RUSSIA
Author
Ramzia Lefebvre
Technical Manager for Russia and Customs Union Certification
INTERTEK FRANCE
Government & Trade Services
ABSTRACT
The expansion of the cosmetic and personal care market to all areas of the globe is indicative of the thirst of the peoples of the earth to look good, feel good, and have a healthy aging process. New markets beckon the world’s ingredient developers, marketers, and product manufacturers for the need to expand into these “new” markets. Along with the development of new products for new needs, safety issues and regulations have proliferated. The bottom line here is that if a company wants to sell in a far-flung market, it must become knowledgeable about the changing requirements being promulgated by various countries. One such “new” market is Russia. The Russian and Customs Union markets are highly attractive for cosmetic manufacturers and exporters, as the demand for cosmetic goods increases by 5 or more percent each year. Actually, according to experts of the Russian press agency “Credinform,” the annual growth rate could exceed 15% until 2015. As stated in the agency’s study, import growth is associated not only with a steady growth in demand for foreign-made cosmetics, but also with a reduction of customs duty on imported products after Russia’s WTO accession. The Russian market for cosmetics is one of the most dynamic in the world.
In this chapter we provide a detailed description of requirements for companies wishing to gain a part of this lucrative market, and have designed our description in the form of a series of questions and answers relevant to those seeking access to the expanded Russian market.
2.3.1.2 What is the customs union and what is its aim?
1. Definition of perfumes and cosmetics according to CU Technical Regulation
2. Conformity assessment documents
b. TR declaration of conformity to customs union
3. Requirements for perfumes and cosmetics
2.3.1.5 How different are the new rules from european requirements?
2.3.1.7 Business climate in russia and reforms—russia joined WTO
Much anticipated by the cosmetic world and generating many questions during the two last years, new Technical Regulation No. TR TC 009/2011 on the “Safety of perfumery and cosmetic products” (approved on 23.09.2011 by Customs Union Commission’s Decree no. 799) finally came into force on July 1, 2012. This document became applicable within the whole Customs Union of Russia, Belarus, and Kazakhstan.
We will discuss the general objectives of the Customs Union (hereafter CU) and the differences between the “old” procedure and the new one. We will address whether the new rules simplify access to Russian and CU markets; how the new rules are different from European requirements; and how they will affect exports of cosmetics to Russia.
2.3.1.2 WHAT IS THE CUSTOMS UNION AND WHAT IS ITS AIM?
The new regulation does not concern only Russia, but the whole territory of the CU.
The Customs Union between Russia, Kazakhstan, and Belarus came into existence on January 1, 2010. These three states became one region by economic integration and removal of customs borders between Belarus, Kazakhstan, and Russia beginning on July 1, 2010. During the first stage of the integration process, the aim of the Customs Union was to create:
- ● Common customs tariffs without customs duties inside member-states
- ● Common customs code
- ● Other joint measures for foreign trade regulations
From January 1, 2012, the three states moved to the next step and established a single economic area through a number of inter-state agreements such as: “On single principles and rules of technical regulation”; “On single principles of regulating intellectual property rights protection”; “On coordinated macro-economic policy”; “On single principles and rules of competition” and others. These principles were established in order to build a single market with a single macroeconomic policy that would simplify goods, services, capital, and manpower circulation.
The first joint document of the CU was issued in 2010, establishing unified hygienic requirements and unified assessment rules for various goods, including cosmetics: “Unified Sanitary-epidemiological requirements of the Customs Union” (also known as Decree no. 299 dated 28.05.2010). This document set up mandatory State Registration for the Customs Union instead of previous sanitary-epidemiological legislation in Russia.
Development and approval of the Technical Regulations of Customs Union for different categories of products, including cosmetics, have been the continuity of this reform.
Before July 1, 2012, the majority of cosmetic products (with the exception of fragrances) were subject to a two-stage conformity assessment:
- 1. Customs Union State Registration
- 2. GOST R Declaration of Conformity of Russia or GOST R Customs Union Certificate of Conformity
with slight difference for fragrances, which used to be subject to:
- 1. Customs Union Sanitary Control (testing according single CU requirements);
- 2. GOST R Declaration of Conformity of Russia or GOST R Customs Union Certificate of Conformity.
Both State Registration and Sanitary Control were done according to Unified Sanitary Requirements of the Customs Union (Decree no. 299).
Once the products passed the second step, i.e., GOST R certification, they used to be marked by the GOST R Mark of Conformity to demonstrate product compliance with the applicable Russian standards.
Relevant authorities for both steps of certification were:
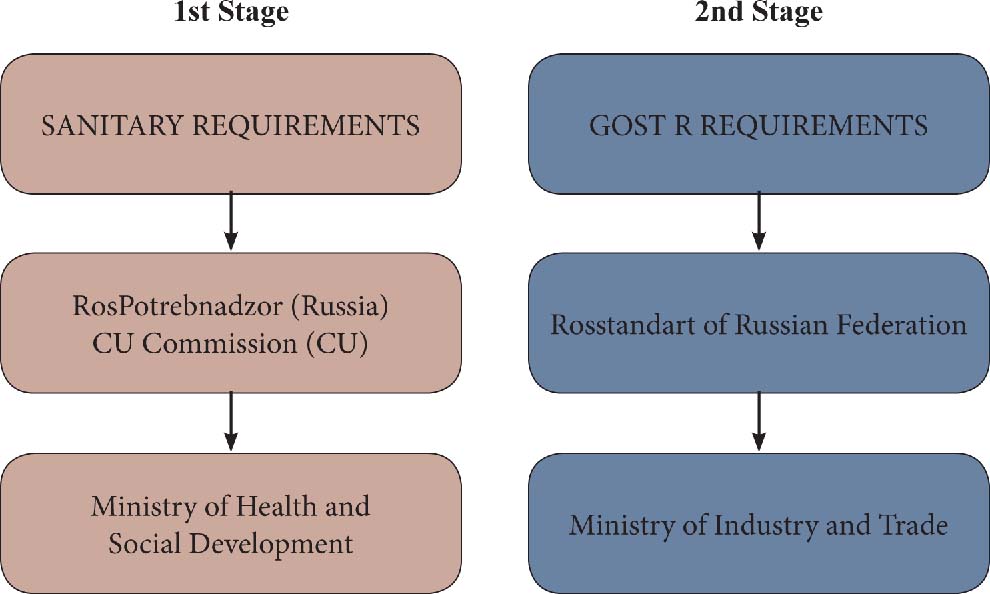
The double conformity assessment takes its origin from the requirements for the Sanitary and GOST, provided by the Ministry of Health and Social Development and the Ministry of Industry and Trade respectively. The basic concern of both authorities and of the Russian certification system was to protect the safety, health, and environment of the Russian people and to prevent dangerous and noncompliant imported products from being placed on the Russian market. Consequently many products entering the territory of the Russian Federation required double conformity assessment in order to:
- 1. Confirm that the products met Russian safety standards relative to the environment, life, and health of population
And in practice to:
- 1. Enable goods pass to through Russian customs
- 2. Enable goods to be used, sold, or marketed on Russian territory
Assessment documents issued according to the old legislation are still valid until July 1, 2014.
With introduction of the new Technical Regulation as of July 1, 2012, the function and aim of assessment documents remain the same. By contrast, the dual compliance system has been abolished. As of July 1, 2012, only one of following assessment documents is required:
- ● either CU State Registration
- ● or CU TR Declaration of Conformity
This topic is expounded in the next chapter.
1. Definition of perfumes and cosmetics according to CU Technical Regulation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








