Fig. 25.1
Four quadrants of the abdominal wall
The second way of dividing the abdominal surface is into nine regions:
1.
Left hypochondriac (LH)
2.
Left lumbar (LL)
3.
Left iliac (LI)
4.
Epigastric (E)
5.
Umbilical (U)
6.
Hypogastric (H)
7.
Right hypochondriac (RH)
8.
Right lumbar (RL)
9.
Right iliac (RI)
These regions are formed by two vertical planes and two horizontal planes. The two vertical planes are the lateral lines (LLL and RLL). These lines are dropped from a point halfway between the jugular notch and the acromion process [1]. The two horizontal planes are the transpyloric plane (TPP) and the transtubercular plane (TTP). The tubercles are the tubercles of the iliac crests (Fig. 25.2).
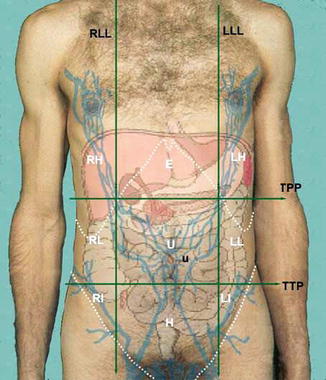

Fig. 25.2
Nine regions of the abdominal wall
The layers of the abdominal wall vary a lot, depending on which side you are analyzing.
The abdominal wall consists of the skin, superficial fascia, fat, muscles, transversalis fascia, and the parietal peritoneum (Fig. 25.3).
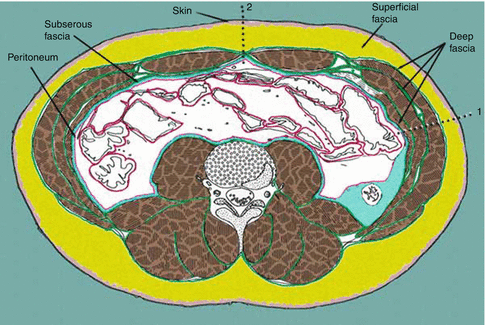

Fig. 25.3
Layers of the anterior abdominal wall. Skin  . Superficial fascia
. Superficial fascia  . Deep fascia
. Deep fascia  . Muscle
. Muscle  . Subserous fascia
. Subserous fascia  . Peritoneum
. Peritoneum  . 1 lateral landamrk of the abdomen, 2 anterior landmark of the abdomen
. 1 lateral landamrk of the abdomen, 2 anterior landmark of the abdomen
 . Superficial fascia
. Superficial fascia  . Deep fascia
. Deep fascia  . Muscle
. Muscle  . Subserous fascia
. Subserous fascia  . Peritoneum
. Peritoneum  . 1 lateral landamrk of the abdomen, 2 anterior landmark of the abdomen
. 1 lateral landamrk of the abdomen, 2 anterior landmark of the abdomenIf we look at the wall inferior to the level of the belly button (umbilicus), we can note that the superficial fascia is divided into two parts:
A superficial fatty part that is continuous with the same layer over the rest of the body (often referred to as Camper’s fascia).
A deep membranous layer that is continuous down into the perineum to surround the penis and to form a layer of the scrotum (Scarpa’s fascia). It contains more fibrous tissue (Fig. 25.4).
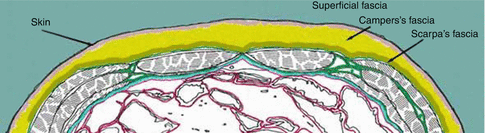
Fig. 25.4
Layers of the anterior-inferior abdominal wall
The blood supply to the superficial layers is derived from branches of the femoral artery, namely, the superficial epigastric arteries. Venous drainage into the femoral veins is facilitated via the saphenous hiatus in the thigh.
Three major arterial branches supply blood to either side of the anterior abdominal wall, which includes two branches of the external iliac artery and a branch of the internal thoracic artery.
The inferior epigastric artery travels within the transversalis fascia until it reaches the arcuate line where it pierces the rectus sheath. The second branch of the external iliac, the deep circumflex iliac, runs parallel to the inguinal ligament between the transversus abdominis and internal oblique muscles. The superior epigastric, the terminal branch of the internal thoracic artery, enters the rectus sheath superiorly.
The cutaneous abdominal wall innervation is consistent with a segmental dermatomal pattern. The anterior and lateral cutaneous branches of the ventral rami of the 7th to 12th intercostal nerves and the ventral rami of the first and second lumbar nerves have important sensory and motor functions. T7 passes to the area just below the infrasternal notch, T10 toward the umbilicus, and T12 to an area just below the umbilicus. The anterior primary rami of this nerve group innervate the abdominal wall musculature as well as the intercostal muscles. There is poor communi cation between nerves as they run toward the midline.
This results in the ability to use transverse incisions through the rectus to gain access to abdominal contents. By reflecting the superficial fascia, the ilioinguinal (L1) and iliohypogastric (T12, L1) nerves can be noted. The iliohypogastric nerve innervates the skin just above the pubis after traversing the external oblique [2].
25.1.1 Anterior Musculature and Ligaments
Much of the strength of the abdominal wall is inherent in four paired muscles and their respective aponeuroses. These aponeuroses represent sheetlike tendons for the insertion of the lateral muscles and also form the sheath of the rectus abdominis.
From most superficial to deep, the external oblique is the first layer of the lateral muscles.
The largest of the three, the external oblique arises from the lower eighth rib posteriorly to interdigitate with both the serratus and latissimus muscles. The direction of the fibers is approximately horizontal in the uppermost portion only to become oblique in the lowest portions as they fold on themselves to form the inguinal ligament. The inguinal ligament helps to define the myopectineal orifice, which is the area contained deep to the inguinal ligament. After contributing to the anterior portion of the rectus abdominis sheath, the remaining fibers insert onto the linea alba, which is the dense white line formed by the medial termination of all the aponeuroses.
The external and internal oblique muscles both have functions in the support of abdominal viscera as well as assisting in flexion and rotation of the trunk. The internal oblique arises from the anterior two-thirds of the iliac crest and lateral half of the inguinal ligament to run essentially at right angles to those of the external oblique. The fibers take the shape of the iliac crest in that they fan out to insert on the 10th to 12th ribs inferiorly.
Spigelian hernias in adults are considered to be acquired through areas of separation of the internal oblique and transversus fibers. These fibers arch over the spermatic cord (or round ligament) and the most inferior of these join with similar aponeurotic fibers of the transverses abdominis to form the conjoint tendon.
The umbilicus marks an important level in the division of the internal oblique aponeurosis. Above this level, the aponeurosis of the internal oblique splits to envelop the rectus abdominis and subsequently rejoins at the linea alba. The contribution to the linea alba inferior to the umbilicus is somewhat more direct. Here, the aponeurosis remains intact and runs anterior to the rectus to finally contribute to the linea alba. The entire rectus sheath can now be illustrated with the inclusion of the aponeurosis of the transverses abdominis muscle. These lateral muscles are important in the formation of midline incisional hernias. In addition to infection of underlying wounds, incisional hernias are in large part due to disinsertion of these lateral muscles in the midline, resulting in retraction and subsequent atrophy.
The final contribution to the rectus sheath arises from the innermost of the three lateral abdominal muscles, the transversus abdominis. These muscles arise from the 7th to 12th costal cartilages, iliac crest, and the lateral third of the inguinal ligament. The muscle bundles of lower most medial fibers run a more inferomedial course to their insertion on the pubic crest and pectin pubis.
The umbilicus is an important landmark in the division of the transversus abdominis muscle fibers. Above the umbilicus the transversus abdominis aponeurosis joins the internal oblique aponeurosis to form a portion of the posterior rectus sheath as mentioned previously. Below the umbilicus, the transversus aponeurosis only contributes to the anterior rectus sheath. The arcuate line (of Douglas) is the site at which termination of these contributing fibers onto the posterior aspect of the rectus abdominis muscle occurs.
The principal vertical muscle of the anterior abdominal wall consists of a pair of muscles separated by the linea alba. The rectus abdominis muscle originates from the 5th through 7th costal cartilages to insert on the symphysis pubis and crest. Superiorly, the rectus is wide, broad, and thin, becoming narrow and thick inferiorly. The rectus muscle and sheath form the linea semilunaris laterally. Segmentation of each rectus muscle occurs by tendinous intersections that represent attachment of the rectus muscle with the anterior layer of the rectus sheath. In 80 % of people there is a small triangular muscle, called the pyramidalis, located anterior to the inferior part of the rectus. It assists in tensing the linea alba.
The superior and inferior epigastric arteries are the principal blood supply to the rectus.
Laterally, the 7th through the 12th intercostal nerves provide innervation. The rectus abdominis is therefore invested within a sheath derived from the combined aponeuroses and fasciae of the external oblique, internal oblique, and transversus abdominis. Further delineation of the rectus sheath is important in the understanding of anterior abdominal wall anatomy.
The rectus sheath has contributions from all the aforementioned aponeuroses only when inferior to the umbilicus.
The anterior sheath superior to the umbilicus is composed only of aponeuroses from the external and internal abdominal muscles; the transversalis aponeurosis has no contribution to the formation of the anterior sheath at this level. In effect, the internal oblique aponeurosis splits, allowing one layer to pass anterior and one posterior to the rectus muscle. The anterior layer will then join with the external oblique aponeurosis to form the anterior wall of the rectus sheath.
The anterior sheath can be truly considered a composite of all three aponeurotic layers only at a variable level below the umbilicus. The posterior sheath is similarly described in relation to the umbilicus. Superior to the umbilicus, the posterior sheath consists of contributions from both the aponeuroses of the internal oblique and the transversus abdominis. Inferior to the umbilicus, the external abdominal aponeurosis has no contribution to the formation of the posterior rectus sheath.
Bleichrodt’s group has taken advantage of these aponeurotic layers to modify the “component separation” technique. They use this technique to close abdominal defects by finely choosing muscle layers and their investing aponeurosis while simultaneously creating a smaller wound surface.
The arcuate line is defined by the most inferior extension of the posterior sheath forming a crescent-shaped border. In the midline, fibers of the anterior and posterior sheaths interlace forming the linea alba. It is now recognized that mechanical forces acting here contribute to the formation of epigastric hernias.
25.2 Complications in Liver and Kidney Transplantation
Over the last 20 years, transplant patients have significantly improved their long-term survival and outcomes, according to the advances in surgical techniques, the postoperative care, and the use of new immunosuppressive therapies. Nevertheless this complex procedure is often associated with a variety of surgical complications that contribute to patient morbidity and mortality.
Studies that have examined long-term outcomes after liver transplantation indicate that approximately 5–30 % of recipients will develop vascular or biliary complications after transplantation. Although these complications can occur both early (<3 months) and late (>3 months) after transplantation, there are often differences in the pathogenesis and presentation of them. Early complications may result from imperfect surgical techniques and can be dramatic in presentation; instead late surgical complications are typically due to protracted ischemic or immunogenic injury to the graft and often have a more indolent course.
The most common general surgical complication after liver transplantation is herniation. Incisional hernias are by far the most frequent hernia type, occurring in 5–25 % of patients [3]. Risk factors implicated in the development of incisional hernias include obesity, sirolimus-based immunosuppression therapy, and steroid boluses used to treat rejection. Incisional hernias can be observed in high-risk patients and can be successfully repaired with either open or laparoscopic herniorrhaphy in symptomatic patients; repair with a prosthetic mesh yields the lowest recurrence rate. Right-sided diaphragmatic hernias have also been observed in children following liver transplantation. Although the incidence of this complication is very low (<1 %), surgical repair is recommended as these hernias appear to be more frequently associated with bowel obstruction.
Bowel obstruction is less common than herniation in liver transplant recipients (1–2 %) but still remains an important source of morbidity. Bowel obstructions due to intraperitoneal adhesions, internal hernias, abdominal wall hernias (both incisional and inguinal), and neoplasms (including posttransplant lymphoproliferative disease) have all been reported [4]. Notably, bowel obstructions in patients reconstructed with a Roux-en-Y choledochojejunostomy can be caused by herniation of a bowel segment through the mesenteric defect of the Roux limb. Thus, a high degree of suspicion for this specific complication must be maintained in patients who develop a bowel obstruction in the setting of a previous choledochojejunostomy. These patients can present with bowel strangulation in the absence of radiographic bowel obstruction; a contrast CT scan is often diagnostic of this life-threatening complication. Different is the incidence of incisional hernia in kidney-transplanted patient whose percentage is approximately 3 % [5].
In both classes of patients, obesity (body mass index (BMI) ≥30), increased age (over 50 years), use of immunosuppressive therapy, and surgical complications were identified as the most important predisposing factors for incisional hernias. BMI in fact increases the risk of incisional hernia whom percentage is around 11 % [6–8]. However, in patients undergoing liver transplant and immunosuppressive therapy based on the use of mTOR, the incidence rates of incisional hernia is rising to 54.4 %.
Therefore, wound complications are important causes of early and late postoperative morbidity following laparotomy in transplanted patients. Surgical wounds in normal, healthy individuals heal through an orderly sequence of physiologic events that include inflammation, epithelialization, fibroplasia, and maturation. Mechanical failure or failure of wound healing at the surgical site can lead to disruption of the closure leading to seroma, hematoma, wound dehiscence, or hernia. Other complications include surgical site infection and nerve injury. The ideal abdominal wound closure provides strength and a barrier to infection. In addition, the closure should be efficient, performed without tension or ischemia, comfortable for the patient, and aesthetic.
The incisional hernia has been defined as a bulge visible and palpable when the patient is standing and often requiring support or repair. This common complication of abdominal surgery is reported in up to 11 % of patients generally and in up to 23 % of those who develop postoperative wound infection.
A considerable proportion of patients present with incarceration and strangulation, requiring emergency abdominal surgery. Treatment involves further major surgery, and the results may be poor, with recurrence rates up to 49 % [9]. These high recurrence rates prompted recommendations of a caution attitude to surgical treatment of incisional hernia in the mid-1980s [10]. Since then, in spite of the frequency of the condition and its potential morbidity, no consensus on the best treatment has been forthcoming [11].
A wide spectrum of surgical techniques has been developed and recommended with varying results, ranged from sutured techniques to the use of various types of prosthetic mesh. Laparoscopic repair was a novel approach introduced in the 1990s. However, the gold standard has not yet been found (Table 25.1).
Table 25.1
European Hernia Society classification for incisional abdominal wall hernias
EHS Incisional hernia classification | |||
|---|---|---|---|
Midline | Subxiphoidal | M1 | |
Epigastric | M2 | ||
Umbilical | M3 | ||
Infraumbilical | M4 | ||
Suprapubic | M5 | ||
Lateral | Subcostal | L1 | |
Flank | L2 | ||
Iliac | L3 | ||
Lumber | L4 | ||
Recurrent incisional hernia? | Yes ◯ | No ◯ | |
Length: cm | Width: cm | ||
Width cm | W1 <4 ◯ | W2 ≥4–10 ◯ | W3 ≥10 ◯ |
Modern thinking suggests that the surgeon needs to accomplish all the following four goals to create the strongest ventral hernia repair (Fig. 25.5):
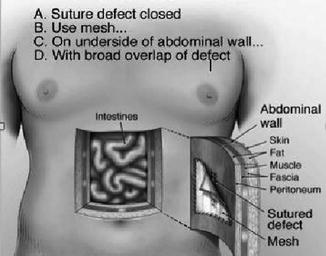

Fig. 25.5
Strongest hernia repair
1.
Suture the closed defect. For a variety of reasons, this is not always achieved. However, it is one of the four required components if the strongest repair is to be accomplished.
2.
Reinforce the repair with mesh. It looks much like window screen and allows the body to grow into the spaces within the mesh. This ingrowth of tissue fuses the mesh to the abdominal wall adding strength/reinforcement to what would otherwise be an “un-reinforced” sutured repair. The fusion process begins immediately following the operation and continues for a number of months.
3.
Place the mesh inside the abdomen on the underside of the abdominal wall (underlay repair). Placed in this location, pressures inside the abdomen (including those caused by physical activity) force the mesh against the underside of the abdominal wall, facilitating the body’s ingrowth/fusion into the mesh.
4.
Achieve the broadest possible contact of mesh with the abdominal wall. The strength of any mesh repair is determined by the amount of that mesh that is available for the abdominal wall to grow into. This overlap beyond the edges of the defect should be a minimum of 2–3 inches because the larger the surface area of fusion between the abdominal wall and the mesh, the stronger the hernia repair.
25.3 Open Repair Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree