Sexually Transmitted Diseases 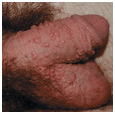
Etiology and Epidemiology
Etiology. HPV is DNA papovavirus that multiplies in the nuclei of infected epithelial cells (see Section 27). More than 20 types of HPV can infect the genital tract: types 6, 11 most commonly. Types 16, 18, 31, 33, and 35 are strongly associated with anogenital dysplasia and carcinoma. In persons with multiple sexual partners, subclinical infection with multiple HPV types is common.
Risk Factors for Acquiring HPV Infection. Number of sexual partners/frequency of sexual intercourse. Sexual partner with HPV anogenital infection. Infection with other STIs.
Transmission. Through sexual contact: genital-genital, oral-genital, genital-anal. Microabrasions occur on epithelial surface allowing virions from infected partner to gain access to basal cell layer of noninfected partner.
• During delivery, mothers with anogenital warts can transmit HPV to neonate, resulting in EGW and laryngeal papillomatosis in children.
Incidence. Most sexually active individuals are subclinically infected with HPV; most HPV infections are asymptomatic, subclinical, or unrecognized. 1% of sexually active adults (15-19 years of age) develop clinical lesions.
Pathogenesis. “Low-risk” and “high-risk” HPV types both cause anogenital infections. HPV infection may persist for years in a dormant state and becomes infectious intermittently. Exophytic warts are probably more infectious than subclinical infection. Immunosuppression may result in new extensive HPV lesions, poor response to treatment, increased multifocal intraepithelial neoplasia. All HPV types replicate exclusively in host’s cell nucleus. In benign HPV-associated lesions, HPV exists as a plasmid in cellular cytoplasm, replicating extrachromosomally. In malignant HPV-associated lesions, HPV integrates into host’s chromosome, following a break in the viral genome (around E1/E2 region). E1 and E2 function is deregulated, resulting in cellular transformation.
Clinical Manifestation
Usually asymptomatic, except for cosmetic appearance. Anxiety of having STI. Obstruction if large mass is uncommon.
Mucocutaneous Lesions. Four clinical types of genital warts occur:
Small papular (Fig. 30-1).
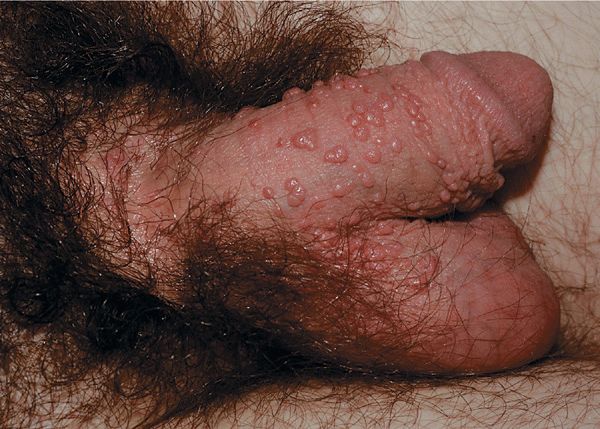
Figure 30-1. Papular warts: penis A 23-year-old male with penile lesions for 6 months. Multiple skin-colored papules on the penis and scrotum.
Condyloma acuminatum. Cauliflower-floret (acuminate or pointed) lesions (Figs. 30-2 to 30-5).
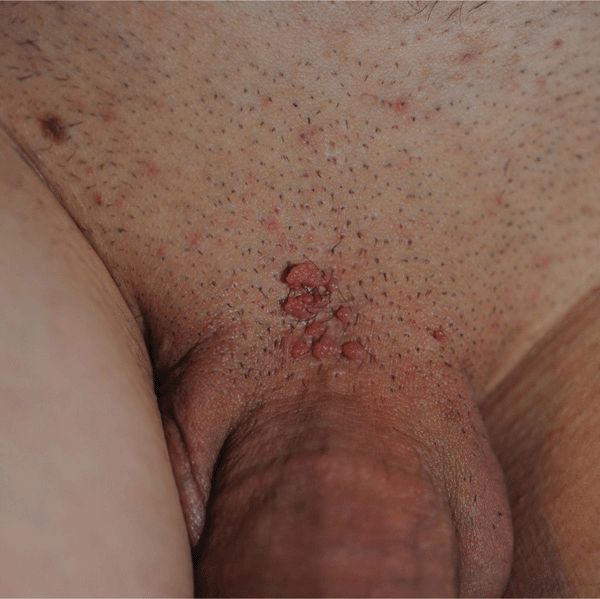
Figure 30-2. Condyloma acuminatum A 30-year-old male with cluster of warts at the base of the penis in pubis for 6 weeks. This is a common site for HPV infection; condom use does not protect against transmission from infected partner.
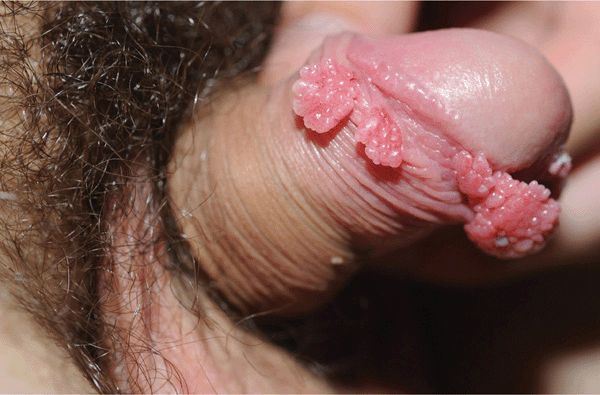
Figure 30-3. Condylomata acuminata: penis A 20-year-old male with Crohn disease treated with infliximab infusion. Condylomata on the distal foreskin resemble cauliflower floret-like papules.
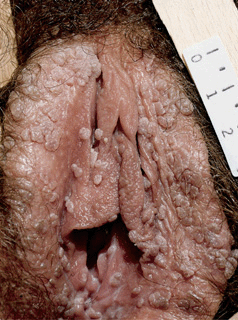
Figure 30-4. Condylomata acuminata: vulva Multiple, pink-brown, soft papules on the labia.
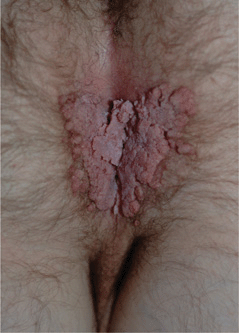
Figure 30-5. Genital warts A 37-year-old male with history of heart-lung transplantation and immunosuppression. Large condylomata acuminata are seen on the anal and perineal area.
Keratotic warts (Fig. 30-6).
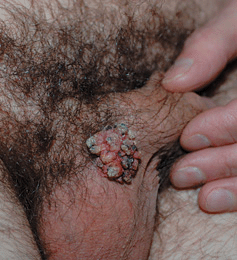
Figure 30-6. Keratotic external genital warts (EGW): male A 46-year-old male with lesion at the base of penis for several years. A keratotic tumor at the base of the penis adjacent to the scrotum. Lesional biopsy reported EGW ruling out verrucous carcinoma.
Flat-topped papules/plaques (most common on cervix) (Fig. 30-7).
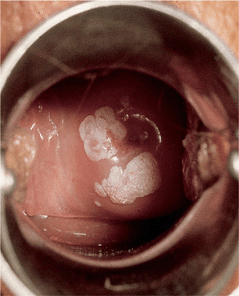
Figure 30-7. Condylomata acuminata: uterine cervix Sharply demarcated, whitish, flat plaques becoming confluent around the cervix.
Skin-colored, pink, red, tan, brown. Solitary, scattered, and isolated, or form voluminous confluent masses. In immunocompromised individuals, lesions may be huge (Fig. 30-5).
Sites of predilection. Male: Frenulum, corona, glans penis, prepuce, shaft (Figs. 30-1, 30-2, 30-5, 30-6), scrotum. Female: Labia, clitoris, periurethral area, perineum, vagina, cervix (flat lesions) (Fig. 30-7). Both sexes: Perineal, perianal (Fig. 30-5), anal canal, rectal; urethral meatus, urethra, bladder; oropharynx.
Laryngeal Papillomas
• Relatively uncommon; associated with HPV-6 and -11.
• Arise most commonly on true vocal cords of larynx.
• Age: children <5 years of age, adults >20 years of age.
• Risk of SCCIS and invasive SCC.
Differential Diagnosis
Papular/Nodular External Genital Lesions. Normal anatomy (e.g., sebaceous glands, pearly penile papules, vestibular papillae), squamous intraepithelial lesions (SILs), SCCIS, invasive SCC, benign neoplasms (moles, seborrheic keratoses, skin tags, pilar cyst, angiokeratoma), inflammatory dermatoses (lichen nitidus, lichen planus), molluscum contagiosum, condylomata lata, folliculitis, scabietic nodules.
Laboratory Examinations
Pap Smear. Encourage all women to have annual Pap smear since HPV is the major etiologic agent for cancer of the cervix. Anal Pap test with a cervical brush and fixative solution helps detect anal dysplasia.
Dermatopathology. Biopsy is indicated if diagnosis is uncertain; lesions do not respond to standard therapy and worsen during therapy; the patient is immunocompromised; warts are pigmented, indurated, fixed, and/or ulcerated. Indicated in some cases to confirm diagnosis and/or rule out SCCIS or invasive SCC.
Detection of HPV DNA. Presence of HPV DNA and specific HPV types determined on smears and lesional biopsy by in situ hybridization. Serology. Genital warts are markers of unsafe sexual practices. Serologic tests for syphilis should be obtained on all patients to rule out coinfection with Treponema pallidum, and all patients offered HIV/AIDS testing.
Diagnosis
Clinical diagnosis, occasionally confirmed by biopsy.
Course
HPV is highly infectious, with an incubation period of 3 weeks to 8 months. Most HPV-infected individuals who develop genital warts do so 2-3 months after becoming infected. If left untreated, genital warts may resolve on their own, remain unchanged, or grow. After regression, subclinical infection may persist for life. Recurrence may occur with normal immune function as well as in immunocompromised. Recurrences more commonly are reactivation of subclinical infection than reinfection. In pregnancy, genital warts may increase in size and number, show increased vaginal involvement, and have an increased rate of secondary bacterial infection. Children delivered vaginally of mothers with genital HPV infection are at risk for developing recurrent respiratory papillomatosis in later life.
HPV types 16, 18, 31, and 33 are the major etiologic factors for in situ and invasive SCC: Cervix; external genitalia (vulva and penis); anus and perineum (homosexual/bisexual males but not necessarily, females).
Management
Prevention. Use of condoms reduces transmission. HPV vaccine protects against four strains of HPV.
Goal of Treatment. Removal of exophytic warts and reduction of signs and symptoms. No therapy has been shown to eradicate HPV or prevent cervical or anogenital cancer. Treatment more successful if warts are small and present for <1 year. Risk of transmission might be reduced by “debulking” genital warts.
Selection of Treatment. Guided by preference of patient—avoid expensive therapies, toxic therapies, and procedures that result in scarring. See Section 27.
Patient Applied Agents. Imiquimod 5% cream, podophylox 0.5% solution.
Clinician Administered Therapy. Cryosurgery, podophyllin 10-25%, trichloroacetic acid 8090%, surgical removal, electrodesiccation.
Etiology and Epidemiology
The Bethesda System (National Cancer Institute) is currently used as terminology for “dysplastic” lesions caused by HPV on anogenital sites. The terminology applies to both cytologic (Pap test) and histologic assessments. Intraepithelial neoplasia are designated as cervical (CIN), vulvar (VIN), penile (PIN), and anal (AIN). VIN is classified as VIN1 (mild dysplasia), VIN2 (moderate dysplasia), VIN3 (severe dysplasia or carcinoma in situ), and VIN3 differentiated type.
Etiology. HPV types 16, 18, 31, and 33.
Transmission. HPV transmitted sexually. Autoinoculation. Rarely, HPV-16 transmitted from mother to newborn with subsequent development on penis.
Demography. Cervical SCC is the second most common female malignancy worldwide, second only to breast cancer. It is the most frequent malignancy in developing countries—500,000 new cases and 200,000 deaths worldwide attributed to it annually.
Risk Factors. Host defense defects and cigarette smoking are risk factors for more dysplastic lesions and invasive SCC.
Pathogenesis. HPV-16- and -18-infected cells may not be able to differentiate fully as a result of either: Functional interference of cell cycle-regulating proteins, caused by viral gene expression or overproduction of E5, E6, and E7. When this occurs, the host DNA synthesis continues unchecked and leads to rapidly dividing undifferentiated cells with morphologic characteristics of intraepithelial neoplasia. Accumulated chromosomal breakages, rearrangements, deletions, and other genomic mutations in these cells lead to cells with invasion capability and, ultimately, to cervical malignancy.
Clinical Manifestation
Prior history of condylomata acuminata. Female partners of males may have CIN.
Mucocutaneous Lesions
• Erythematous flat-topped papules.
• Lichenoid (flat-topped) or pigmented papules (called bowenoid papulosis) (Figs. 30-8, 30-9).
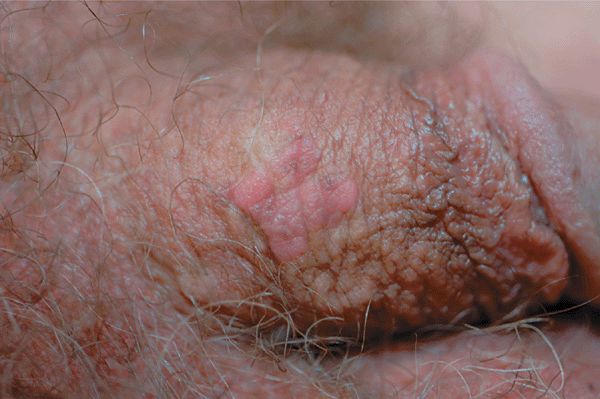
Figure 30-8. HPV squamous cell carcinoma in situ A 48-year-old male with penile lesion for 2 years. Pink papules forming a 1-cm plaque on the shaft of the penis. Lesional biopsy reported SCCIS with HPV changes (koilocytosis).
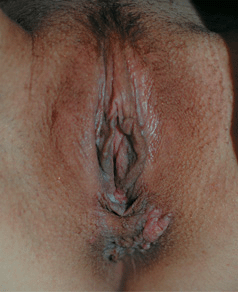
Figure 30-9. HPV squamous cell carcinoma in situ A 33-year-old renal transplant recipient with anogenital lesions for several years. A large pink plaque on the perineum and multiple small papules on posterior vulva. Lesional biopsy was reported to show SCCIS with HPV changes (koilocytosis).
• May show confluence or form plaque(s).
• Leukoplakia-like plaque (Fig. 30-10). Surface usually smooth, velvety.
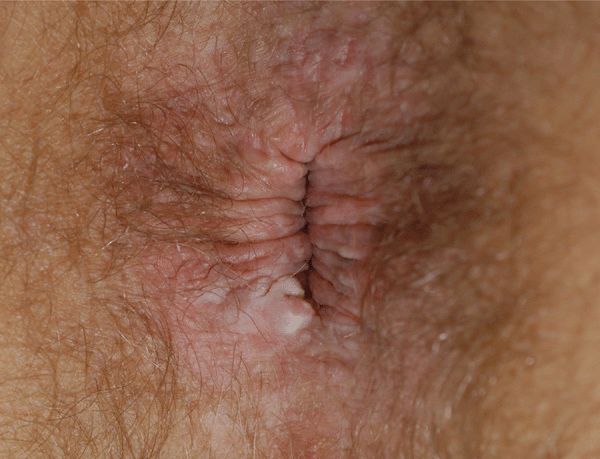
Figure 30-10. HPV squamous cell carcinoma in situ A 49-year-old male with HIV disease noted to have anal lesion for 1 month. A white firm nodule on the rim of the anus. Biopsy reported SCCIS with HPV changes. No lesions were detected on anal colposcopy.
Colors: Tan, brown, pink, red, violaceous, white. Nodule or ulceration in field of SIL suggests invasive SCC (Figs. 30-11 and 30-12).
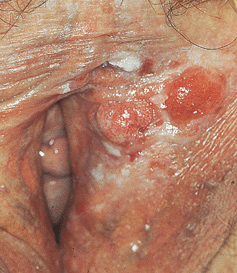
Figure 30-11. HPV-induced in situ and invasive squamous cell carcinoma: vulva Several red nodules (invasive SCC) arising within a white plaque (SCCIS) on the left labium.
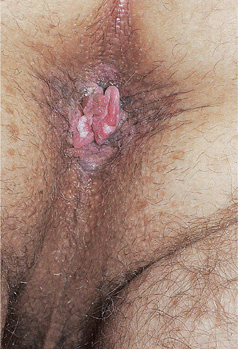
Figure 30-12. HPV-induced in situ and invasive squamous cell carcinoma: perineal/perianal A 38-year-old male with HIV disease aware of perianal lesions for several months; he had prior history of EGW. Brown perineal and perianal macules and papules (SCCIS) with a pink nodule arising at the anal verge. Excisional biopsy of the nodule reported invasive SCC arising within SCCIS.
Arrangement. Characteristically clusters, i.e., commonly multifocal. May be solitary.
Distribution. Males: glans penis, prepuce (75%) (flat lichenoid papules or erythematous macules); penile shaft (25%) (pigmented papules). Females: labia majora and minora, clitoris. Multicentric involvement of the cervix, vulva, perineum, and/or anus occurs not infrequently. Both sexes: inguinal folds, perineal/perianal skin. Oropharyngeal mucosa. Sites other than external genitalia may be associated with cervical dysplasia, CIN, cervical SCC; rarely, SCCIS of other sites, i.e., nail unit (periungual, nail bed); intraoral (Fig. 30-13).
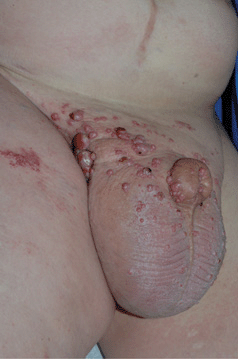
Figure 30-13. Metastatic SCC of urethra A 38-year-old male with primary urethral squamous cell carcinoma metastatic to inguinal lymph nodes with lymphedema. Red nodules and plaques are cutaneous metastases. PCR of thigh metastasis detected HPV-16.
Differential Diagnosis
Multiple Skin-Colored Papules ± Hyperkeratosis. Genital warts, psoriasis vulgaris; lichen planus.
Pigmented Anogenital Macule(s)/Papule(s). Genital lentiginosis, melanoma (in situ or invasive), pigmented basal cell carcinoma, angiokeratomas.
Laboratory Examinations
Dermatopathology. Epidermal proliferation with numerous mitotic figures, abnormal mitoses, atypical pleomorphic cells with large hyperchromatic, often clumped nuclei, dyskeratotic cells; basal membrane intact. Koilocytosis. Recent application of podophyllin to condyloma acuminatum may cause changes similar to SCCIS.
Southern Blot Analysis. Identifies HPV type.
Pap Smear. Koilocytotic atypia.
Exfoliative Cytology. Cervical Pap smears have been recommended annually for women >50 years of age. Cytology of the anal canal may also be helpful in management of individuals with a history of anal HPV infection, especially if immunocompromised (HIV disease, renal transplant recipients). By the Bethesda System, these cytologic findings are reported as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), high-grade (HSIL), and SCC.
Diagnosis
Clinical suspicion, confirmed by biopsy of lesion.
Course
Invasive SCC develops only through well-defined precursor lesions (Figs. 30-11, 30-12). Over time, these lesions can regress, persist, recur, or progress, in some cases to invasive SCC. Natural history of CIN is best studied: progression to invasive SCC occurs in 36% of cases over a 20-year period. Patients with intraepithelial neoplasias, which often occur in immunocompromised individuals, should be followed indefinitely, with monitoring by exfoliative cytology and lesional biopsy specimens.
Laboratory Findings
Colposcopy
The most common indication for colposcopy is abnormal exfoliative cytology. Acetic acid, 3-5%, is applied to the cervix, which causes columnar and abnormal epithelium to become edematous. Abnormal (atypical) epithelium adopts a white or opaque appearance that can be distinguished from the normal pink epithelium. Abnormal epithelium is then biopsied. Colposcopy can also be performed on individuals with abnormal anal exfoliative cytology, and biopsy specimens obtained from abnormal site(s).
Biopsy
In cases of documented SIL or SCCIS, biopsy specimens should be obtained from rapidly enlarging lesions, areas of ulceration or bleeding, exuberant tissue with abnormal vascularity.
Treatment
The only way of possibly reducing the potential risk of invasive SCC is diagnosis and eradication of intraepithelial disease. Because lesions are relatively uncommon, cases are often best managed by a dermatologist with clinical experience in the care of these patients, an oncologic gynecologist, or a colorectal surgeon. If lesion biopsy specimens do not show early invasion, lesions can be treated medically or surgically.
Medical Management
5-Fluorouracil cream has been used but is difficult to use because of erosions. Imiquimod cream 5% is also effective.
Surgical Management
Surgical excision, Mohs surgery, electrosurgery, laser vaporization, cryosurgery.
Etiology and Epidemiology
Etiology. HSV-2 > HSV-1. See also Section 27.
Prevalence. Highly variable. Depends on many factors: country, region of residence, population subgroup, gender, and age. Greater among higher risk sexual behavior groups. Prevalence of HSV-2 seropositivity in general population: United States: 21%; Europe: 8-15%; Africa: 40-50% in 20-year-olds. Strongly associated with age, increasing from negligible levels in children <12 to as high as 80% among higher risk populations. In the United States, approximately one in five adults infected.
Transmission. Usually skin-to-skin contact. Seventy percent of transmission occurs during times of asymptomatic HSV shedding. Transmission rate in discordant couples (one partner infected, the other not) approximately 10% per year; 25% of females become infected, compared with only 4-6% of males. Prior HSV-1 infection is protective; in females with anti-HSV-1 antibodies, 15% become infected with HSV-2, but in those without anti-HSV-1 antibodies, 30% become infected with HSV-2.
Clinical Manifestation
Only 10% of HSV-2 seropositive individuals are aware that symptoms are those of GH. Ninety percent do not recognize symptoms of GH. Most clinical lesions are minor breaks in the mucocutaneous epithelium, presenting as erosion, “abrasions,” fissures. The “classically” described findings are uncommon. Symptoms of aseptic HSV-2 meningitis can occur with primary or recurrent GH.
Primary Genital Herpes. Most individuals with primary infection are asymptomatic. Those with symptoms report fever, headache, malaise, myalgia, peaking within the first 3-4 days after onset of lesions, resolving during the subsequent 3-4 days. Erythematous papules initially evolve to vesicles or pustules, which become eroded as the overlying epidermis sloughs (Figs. 30-14, 30-15). Primary infection occurs anywhere on the anogenital skin, cervix, and anorectal mucosa. Epithelial defects heal in 2-4 weeks, often with resulting postinflammatory hypo- or hyperpigmentation, uncommonly with scarring.
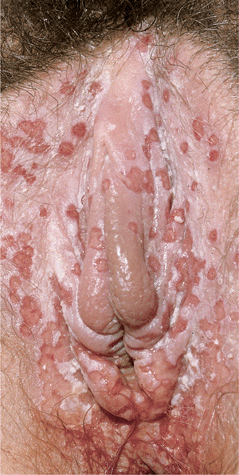
Figure 30-14. Genital herpes, primary Multiple, extremely painful, punched-out, confluent, shallow ulcers on the edematous vulva and perineum. Micturition is often very painful. Associated inguinal lymphadenopathy is common.
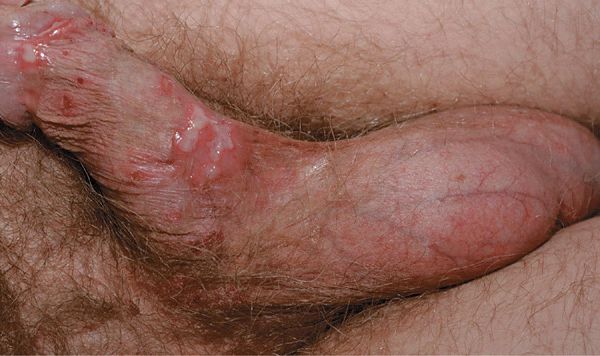
Figure 30-15. Genital herpes, primary A 48-year-old male with painful genital lesions for 4 days. Multiple erosions on the penis and scrotum.
With host defense defects, lesions tend to be more extensive and delayed in healing.
Recurrent Genital Herpes. New symptoms may result from old infections. Most individuals do not experience “classic” findings of grouped vesicles on erythematous base. Common symptoms are itching, burning, fissure, redness, and irritation prior to eruption of vesicles. Dysuria, sciatica, and rectal discomfort. Lesions may be similar to primary infection but on a reduced scale. Often a 1- to 2-cm erythematous plaque with vesicles (Figs. 30-16 to 30-21), which rupture with of erosions.
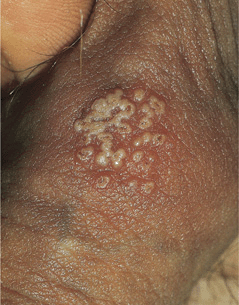
Figure 30-16. Genital herpes, recurrent Group of vesicles with early central crusting on a red base arising on the shaft of the penis. This “textbook” presentation, however, is much less common than small asymptomatic erosions or fissures.
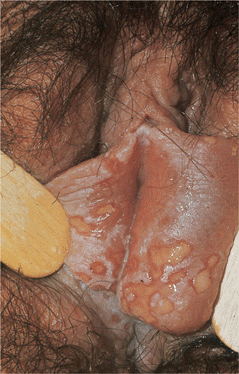
Figure 30-17. Genital herpes, recurrent: vulva Large, painful erosions on the labia. Extensive lesions such as these are uncommon in recurrent genital herpes in an otherwise healthy individual.
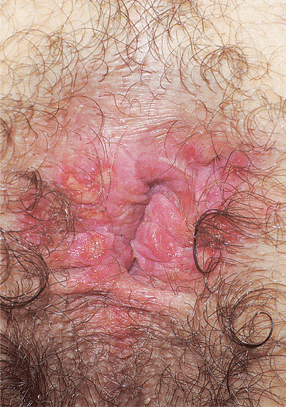
Figure 30-18. Genital herpes, recurrent A 30-year-old male with HIV disease. Multiple, painful, sharply demarcated ulcers are seen on the anus and perineum.
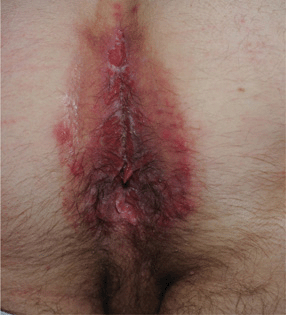
Figure 30-19. Chronic herpetic ulcers A 32-year-old male with extensive painful erosions of perineum and anus. This was the presenting complaint that lead to HIV serotesting and diagnosis of HIV disease.
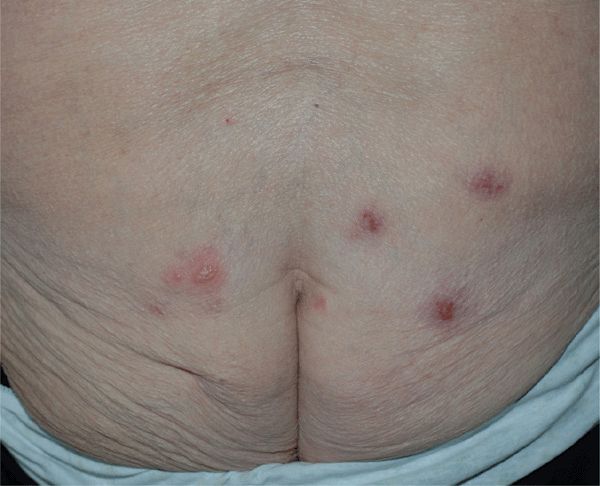
Figure 30-20. Genital herpes, recurrent A 80-year-old female with recurring lesions on buttock. She has polymyalgia rheumatic and is being treated with prednisone. Blisters and crusted erosions are seen on both buttocks.
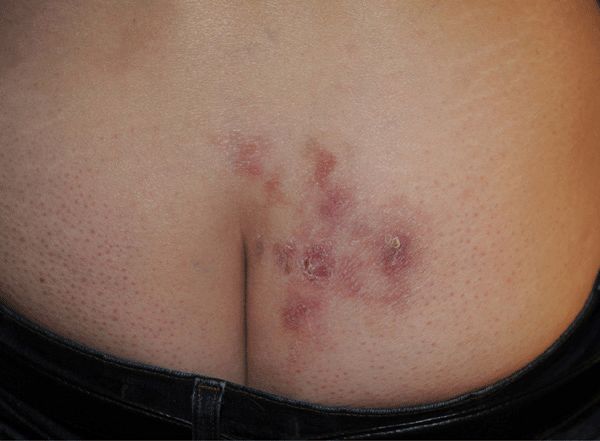
Figure 30-21. Genital herpes, recurrent A 51-year-old female with recurrent vesicles and crusted erosions on buttock, nearly continually since acquisition 31 years before. Recurrent lesion of the buttock were followed by erythema multiforme minor.
Distribution. Males. Primary infection: glans, prepuce, shaft, sulcus, scrotum, thighs, buttocks. Recurrences: penile shaft, glans, buttocks. Females. Primary infection: labia majora/minora, perineum, inner thighs. Recurrences: labia majora/minora, buttocks.
Anorectal Infection. Occurs following anal intercourse; characterized by tenesmus, anal pain, proctitis, discharge, and ulcerations (Figs. 30-18, 30-19) as far as 10 cm into anal canal.
General Findings. Inguinal/femoral lymph nodes may be enlarged, tender with primary infection. Signs of aseptic meningitis. Fever, nuchal rigidity; can occur in the absence of GH. Pain along sciatic nerve.
Differential Diagnosis
Trauma, candidiasis, syphilitic chancre, fixed drug eruption, chancroid, gonococcal erosion.
Laboratory Studies
See Section 27 “Herpes Simplex Virus Disease.”
Diagnosis
Diagnosis can be made on clinical finding. Confirmation by viral culture or direct fluorescent antibody (DFA) or serology may be indicated. Coinfection with another STD should be ruled out.
Course
GH is a lifetime infection and recurrances are the rule. Seventy percent are asymptomatic. Recurrence rates are high in those with an extended first episode of infection, regardless of whether antiviral therapy is given. Chronic suppressive therapy reduces shedding. Treatment of first-episode infection prevents complications such as meningitis and radiculitis. Erythema multiforme may complicate recurrences, occurring 1-2 weeks after an outbreak.
Treatment
Prevention. Advise patients to abstain from sexual activity while lesions are present and encourage use of condoms during all sexual activity.
First Episode. Oral antivirals. Acyclovir 400 mg 5 times daily for 10 days or until lesions resolve.
Recurrances. Oral antivirals. Acyclovir 400 mg 3 times daily for 5 days or 800 mg twice daily for 5 days, or 800 mg 3 times daily for 2 days. Valacyclovir 500 mg twice daily for three days or 1 mg twice daily for 3 days. Famciclovir 125 mg twice daily for 5 days or 1 g once a day for 5 days.
Maintenance Therapy. Oral antivirals: Daily suppressive therapy. Acyclovir 400 mg twice daily. Valcyclovir 500-1000 mg once daily. Famciclovier 250 mg once daily.
Severely Immunocompromised. IV acyclovir 5 mg/kg every 8h for 5-7 days or oral acyclovir 400 mg 5 times a day for 7-14 days.
Acyclovir Resistant. IV foscarnet 40 mg/kg every 8h for 14-21 days.
Neonates. see Section 27.
 ICD-10: B97.7
ICD-10: B97.7 
 Mucosal human papilloma virus (HPV) infections are the most common sexually transmitted infection (STIs) seen by the dermatologist. Only 1-2% of HPV-infected young, sexually active persons have any visibly detectable clinical lesion.
Mucosal human papilloma virus (HPV) infections are the most common sexually transmitted infection (STIs) seen by the dermatologist. Only 1-2% of HPV-infected young, sexually active persons have any visibly detectable clinical lesion. HPV present in the birth canal can be transmitted to a newborn during vaginal delivery and can cause external genital warts (EGW) and respiratory papillomatosis.
HPV present in the birth canal can be transmitted to a newborn during vaginal delivery and can cause external genital warts (EGW) and respiratory papillomatosis. Warts. Barely visible papules to nodules to confluent masses occurring on anogenital skin or mucosa and oral mucosa. EGW: External genitalia, perineum. Cervix. Oropharynx.
Warts. Barely visible papules to nodules to confluent masses occurring on anogenital skin or mucosa and oral mucosa. EGW: External genitalia, perineum. Cervix. Oropharynx. Dysplasia of anogenital and oral skin and mucosa ranging from mild to severe to squamous cell carcinoma (SCC) in situ (SCCIS). Invasive SCC can arise within SCCIS. Most commonly in cervix, anal canal
Dysplasia of anogenital and oral skin and mucosa ranging from mild to severe to squamous cell carcinoma (SCC) in situ (SCCIS). Invasive SCC can arise within SCCIS. Most commonly in cervix, anal canal
 HPV infection of the anogenital epithelium can result in a spectrum of changes referred to as SILs, ranging from mild dysplasia to SCCIS.
HPV infection of the anogenital epithelium can result in a spectrum of changes referred to as SILs, ranging from mild dysplasia to SCCIS. Over time, these lesions can regress, persist, progress, or recur, in some cases to invasive SCC.
Over time, these lesions can regress, persist, progress, or recur, in some cases to invasive SCC. Clinically, lesions appear as multifocal macules, papules, and plaques on the external anogenital region.
Clinically, lesions appear as multifocal macules, papules, and plaques on the external anogenital region. Lesions involving the cervix and anus have the highest risk for transformation to invasive SCC; however, lesions can transform at any site.
Lesions involving the cervix and anus have the highest risk for transformation to invasive SCC; however, lesions can transform at any site. Synonyms: Vulvar intraepithelial neoplasia, penile intraepithelial neoplasm, bowenoid papulosis.
Synonyms: Vulvar intraepithelial neoplasia, penile intraepithelial neoplasm, bowenoid papulosis. ICD-10: A60
ICD-10: A60 
 Genital herpes (GH) is a chronic sexually transmitted viral disease, characterized by symptomatic and asymptomatic viral shedding.
Genital herpes (GH) is a chronic sexually transmitted viral disease, characterized by symptomatic and asymptomatic viral shedding.








