Adverse Cutaneous Drug Reactions1 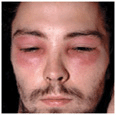
Classification
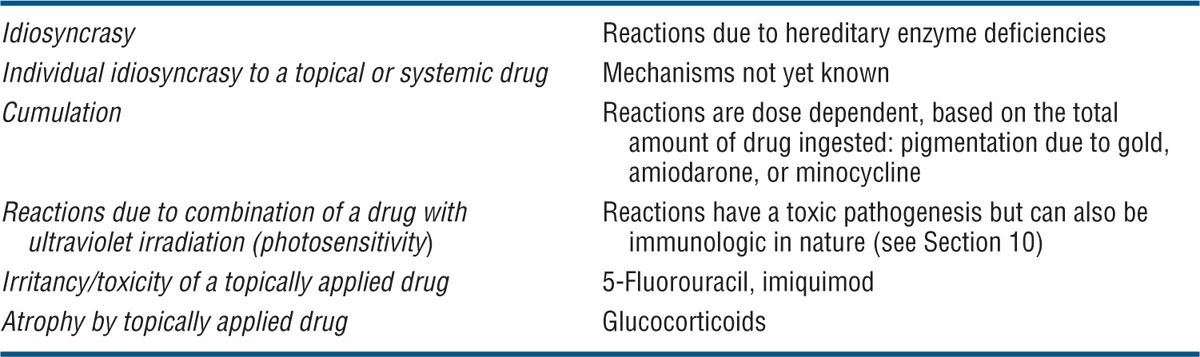
Immunologically Mediated ACDR (see Table 23-1). It should be noted, however, that classification of immunologically mediated ACDR according to the Gell and Coombs classification is an oversimplification because in most reactions both cellular and humoral immune reactions are involved. Nonimmunologic reactions are summarized in Table 23-2.
TABLE 23-1 IMMUNOLOGICALLY MEDIATED ADVERSE CUTANEOUS DRUG REACTIONS*
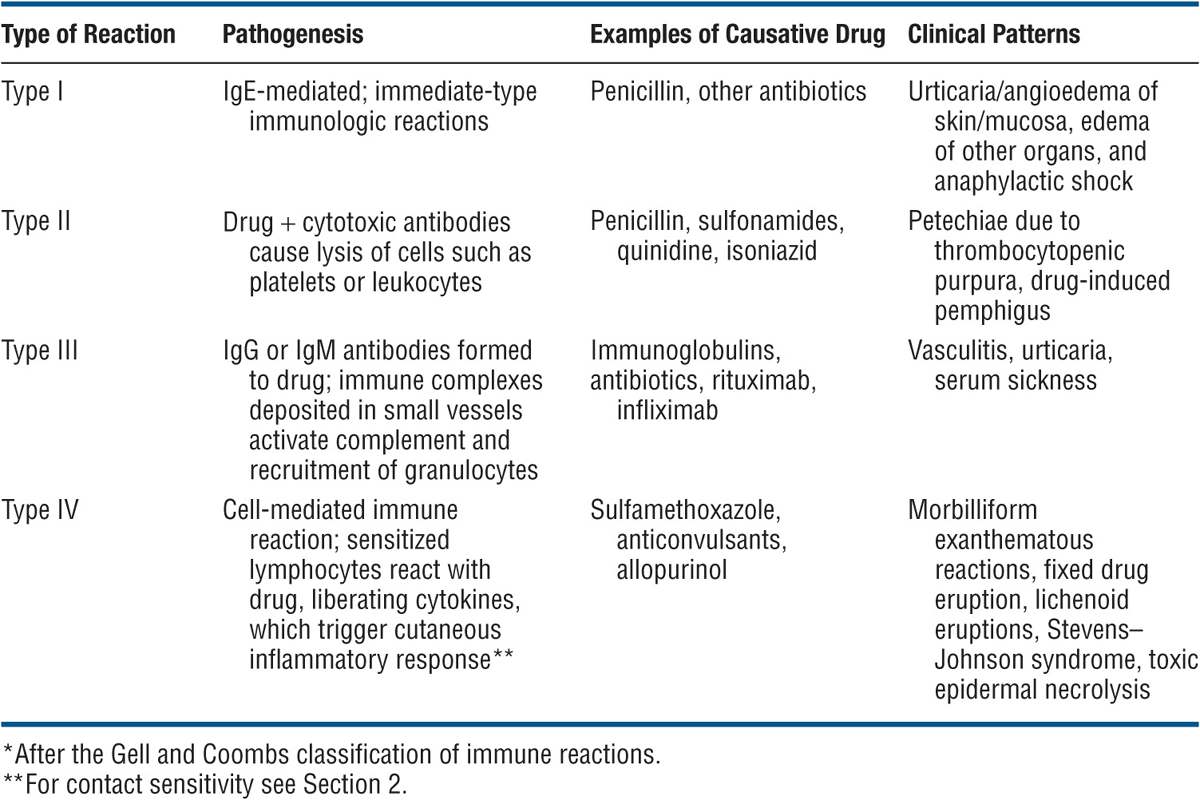
TABLE 23-2 NONIMMUNOLOGIC DRUG REACTIONS
Guidelines for Assessment of Possible ACDRs
• Exclude alternative causes, especially infections, in that many infections (especially viral) are difficult to distinguish clinically from the adverse effects of drugs used to treat infections.
• Examine interval between introduction of a drug and onset of the reaction.
• Note any improvement after drug withdrawal.
• Determine whether similar reactions have been associated with the same compound.
• Note any reaction on readministration of the drug.
Findings Indicating Possible Life-Threatening ACDR
• Skin pain
• Confluent erythema
• Facial edema or central facial involvement
• Palmar/plantar painful erythema
• Concomitant erosive mucous membrane involvement
• Blisters of epidermal detachment
• Positive Nikolsky sign
• Mucous membrane erosions
• Urticaria
• Swelling of the tongue
• High fever (temperature >40°C)
• Enlarged lymph nodes
• Arthralgia
• Shortness of breath, wheezing, hypotension
• Palpable purpura
• Skin necrosis
Clinical Types of Adverse Drug Reactions
ACDRs can be exanthematous and can manifest as urticaria/angioedema, anaphylaxis, and anaphylactoid reactions, or serum sickness; they can mimic other dermatoses; they can present as cutaneous necrosis, pigmentation, alopecia, hypertrichosis; and they can induce nail changes. An overview is presented in Tables 23-3 and 23-4.
TABLE 23-3 TYPES OF CLINICAL ACDRs
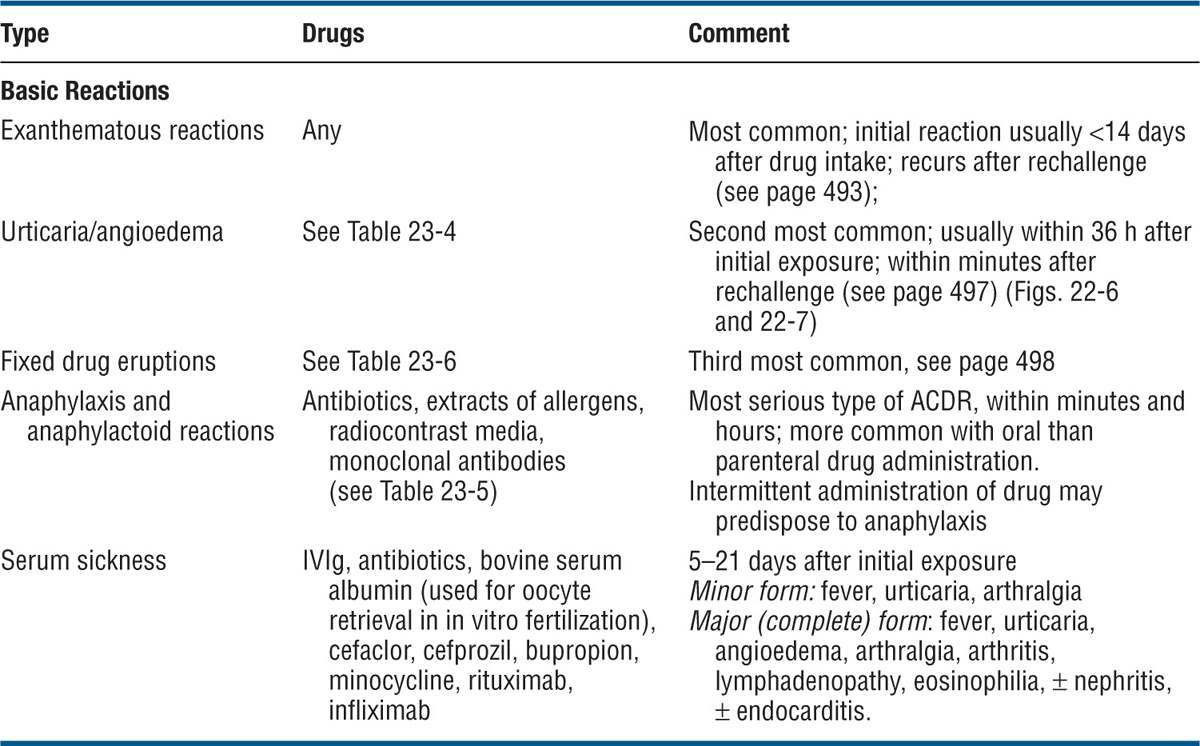
TABLE 23-4 ACDR MIMICRY OF OTHER DERMATOSES
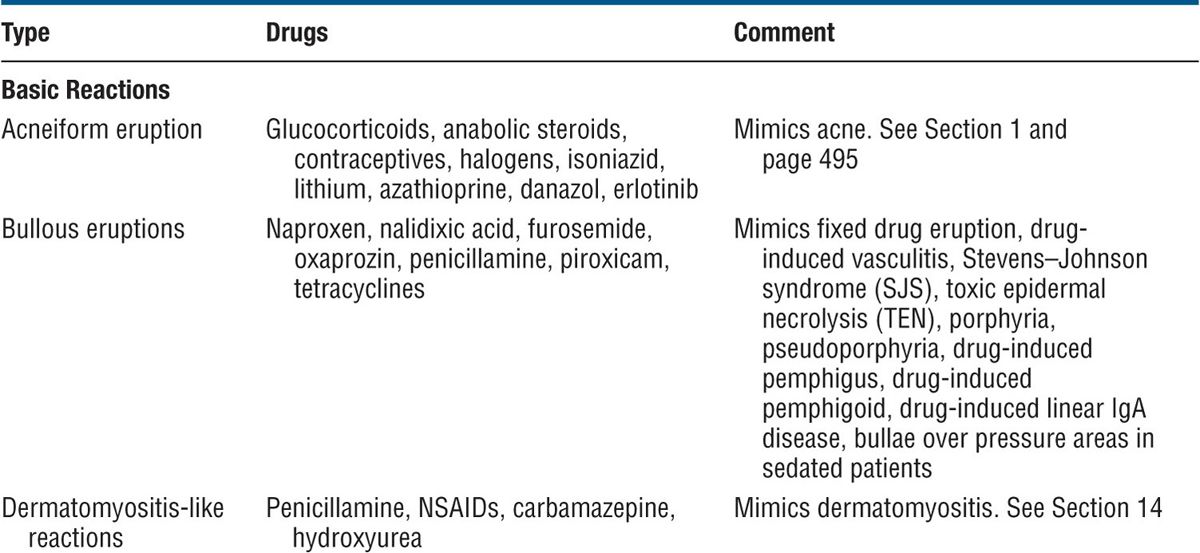
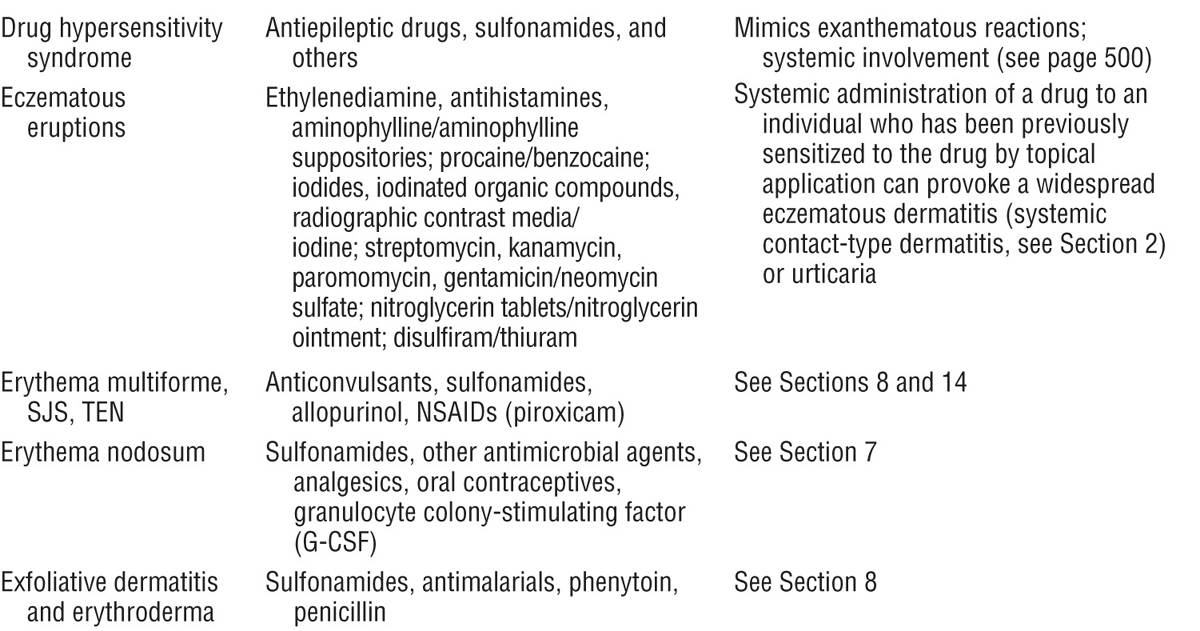
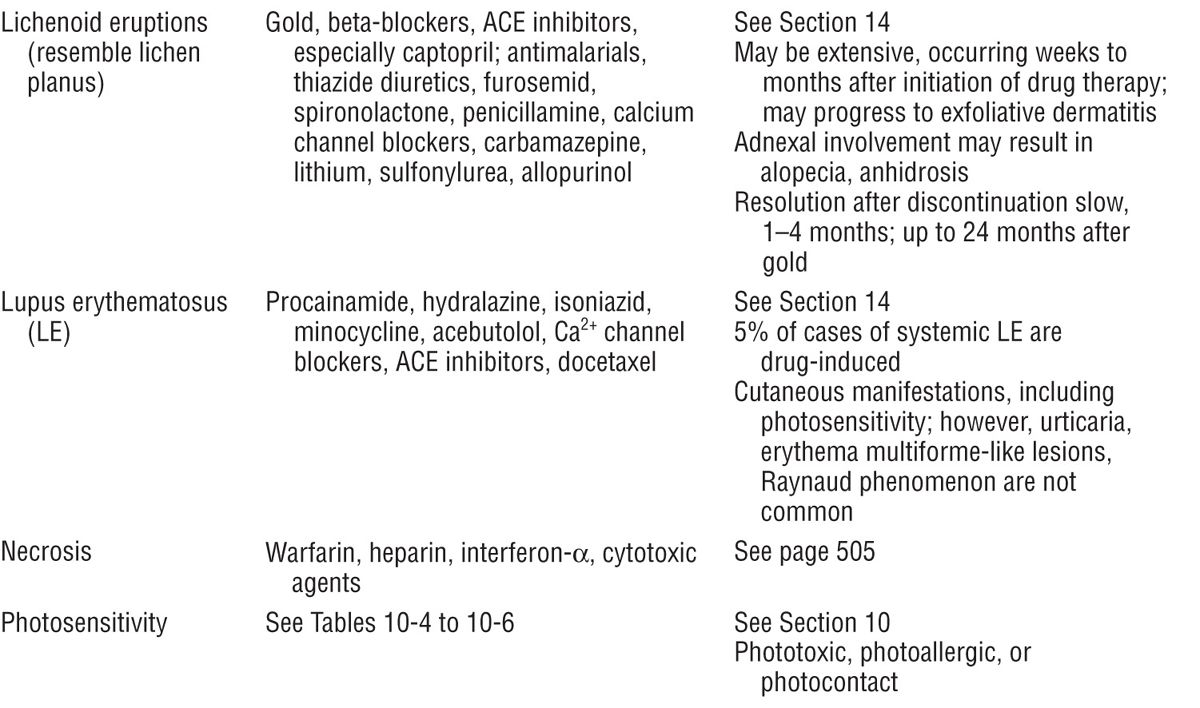
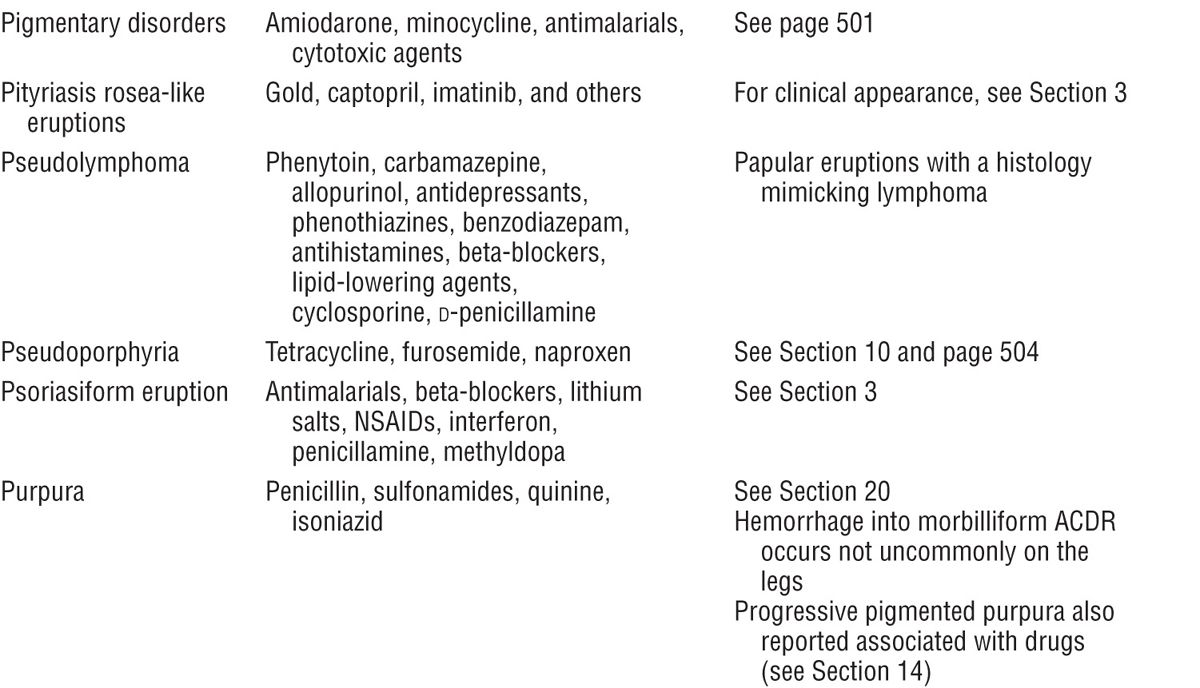
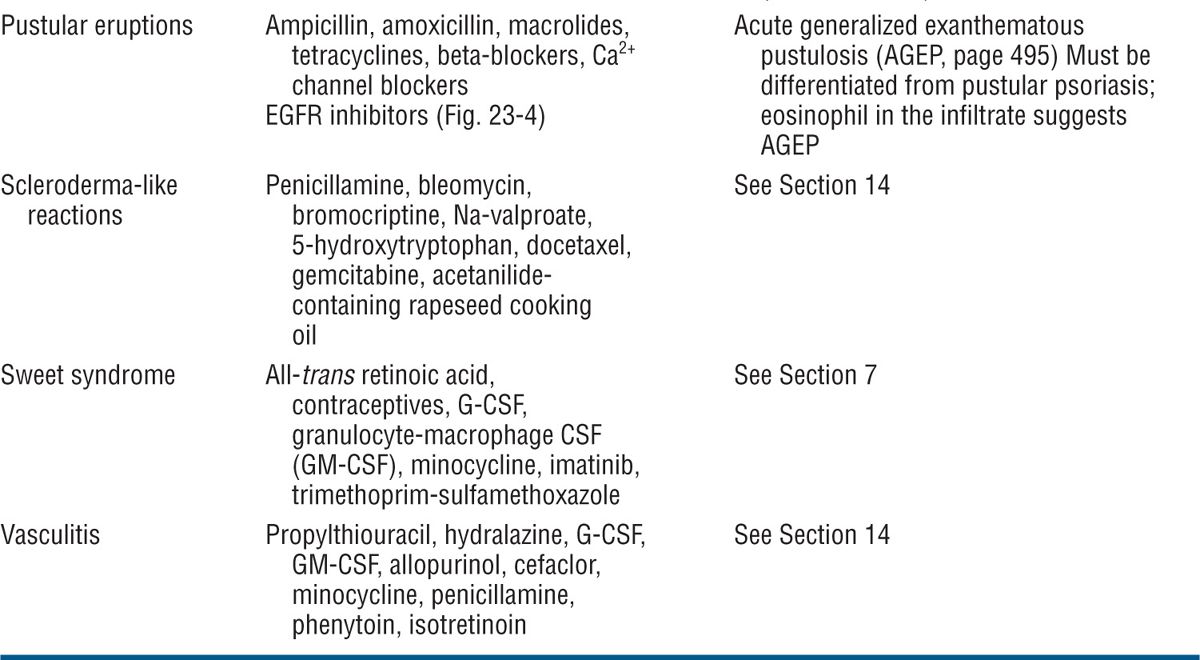
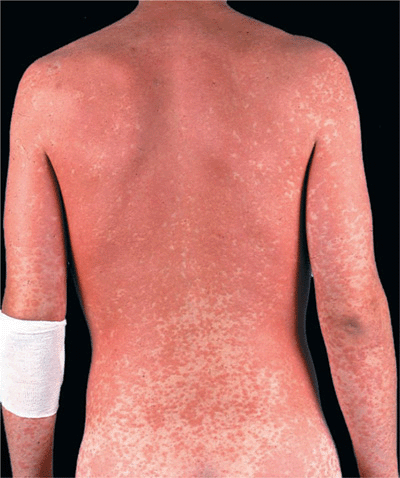
Figure 23-1. Exanthematous drug eruption: ampiciMin Symmetrically arranged, brightly erythematous macules and papules, discrete in some areas, and confluent in others, on the trunk and the extremities.
Reactions to Specific Drugs (Selected)
Allopurinol. Incidence: 5%. Begins on face, spreads rapidly to all areas; may occur in photodistribution. Onset: 2–3 weeks after initiation of therapy. Associated findings: facial edema; systemic vasculitis, especially involving kidneys. Rash may fade in spite of continued administration.
Ampicillin, Amoxicillin. In up to 100% of patients with EBV or CMV mononucleosis syndrome. Increased incidence of EDR to penicillins in patients taking allopurinol. Ten percent cross-react with cephalosporins.
Barbiturates. Site: face, trunk. Onset: few days after initiation of therapy. Cross-reactivity with other barbiturates: not universal.
Benzodiazepines. Rare. Onset: few days after initiation of therapy. Rechallenge: frequently rash does not occur.
Carbamazepine. Morphology: diffuse erythema; severe erythroderma may follow. Site: begins on face, spreads rapidly to all areas; may occur in photodistribution. Onset: 2 weeks after initiation of therapy. Associated findings: facial edema.
Gold Salts. Incidence: 10–20% of patients; dose-related. Morphology: diffuse erythema; exfoliative dermatitis, lichenoid, hemorrhagic, bullous, or pityriasis rosea-like eruptions may follow.
Hydantoin Derivatives. Macular → confluent erythema. Begins on face, spreads to trunk and extremities. Onset: 2 weeks after initiation of therapy. Associated findings: fever, peripheral eosinophilia; facial edema; lymphadenopathy (can mimic lymphoma histologically).
Isoniazid. May evolve to exfoliative dermatitis. Associated findings: fever and hepatitis.
Phenothiazines. Begins on face, spreads to trunk (mainly back), and extremities. Onset: between second and third weeks after initiation of therapy. Associated findings: periorbital edema. Rechallenge: rash may not occur. Cross-reactivity: common.
Sulfonamides. Occurs in up to 50–60% of HIV/AIDS-infected patients (trimethoprim sulfamethoxazole). Patients sensitized to one sulfa-based drug may cross-react with another sulfa drug in 20%.
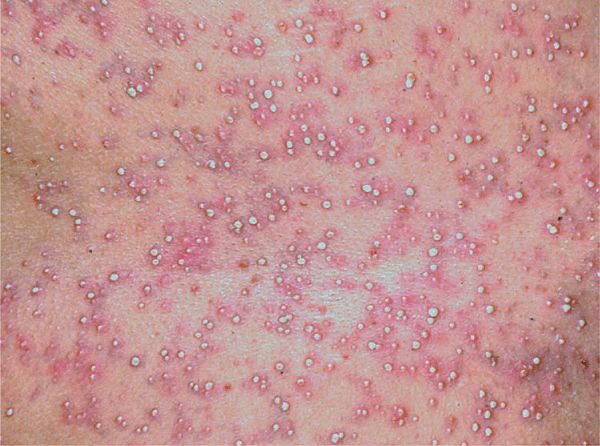
Figure 23-2. Pustular drug eruption: acute generalized exanthematous pustulosis (AGEP) Multiple tiny nonfollicular pustules against the background of diffuse erythema that first appeared in the large folds and then covered the entire trunk and the face.
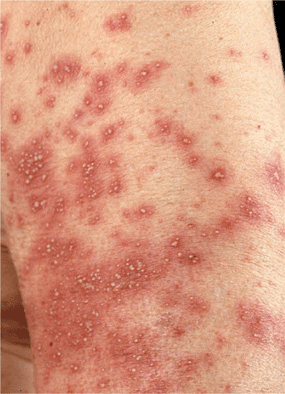
Figure 23-3. Pustular drug eruption: AGEP Multiple sterile pustules surrounded by fiery-red erythema in a 58-year-old female who had fever and leukocytosis. In contrast to the disseminated pustules in Fig. 23-2, here the pustules show a tendency for grouping and confluence. Differential diagnosis of von Zumbusch pustular psoriasis (compare with Fig. 3-13).
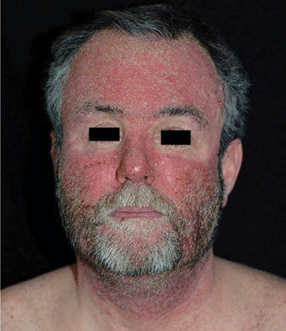
Figure 23-4. Pustular drug eruption: erlotinib This pustular eruption occurred in a patient who had received an anti-EGR monoclonal antibody for cancer of the colon localized to face. Differential diagnosis to acne and rosacea.
 ICD:10: T88.7
ICD:10: T88.7 
 Adverse cutaneous drug reactions (ACDRs) are common in hospitalized (2–3%) as well as in ambulatory patients (>1%).
Adverse cutaneous drug reactions (ACDRs) are common in hospitalized (2–3%) as well as in ambulatory patients (>1%). Most reactions are mild, accompanied by pruritus, and resolve promptly after the offending drug is discontinued.
Most reactions are mild, accompanied by pruritus, and resolve promptly after the offending drug is discontinued. Severe, life-threatening ACDRs do occur and are unpredictable.
Severe, life-threatening ACDRs do occur and are unpredictable. Drug eruptions can mimic virtually all the morphologic expressions in dermatology and must be the first consideration in the differential diagnosis of a suddenly appearing eruption
Drug eruptions can mimic virtually all the morphologic expressions in dermatology and must be the first consideration in the differential diagnosis of a suddenly appearing eruption Drug eruptions are caused by immunologic or nonimmunologic mechanisms and are provoked by systemic or topical administration of a drug.
Drug eruptions are caused by immunologic or nonimmunologic mechanisms and are provoked by systemic or topical administration of a drug. The majority are based on a hypersensitivity mechanism and are thus immunologic and may be of types I, II, III, or IV.
The majority are based on a hypersensitivity mechanism and are thus immunologic and may be of types I, II, III, or IV. ICD-10: T88.7
ICD-10: T88.7 
 An exanthematous drug reaction (EDR) (eruption) is an adverse hypersensitivity reaction to an ingested or parenterally administered drug.
An exanthematous drug reaction (EDR) (eruption) is an adverse hypersensitivity reaction to an ingested or parenterally administered drug. Most common type of cutaneous drug reaction.
Most common type of cutaneous drug reaction. Cutaneous eruption that mimics a measles-like viral exanthem.
Cutaneous eruption that mimics a measles-like viral exanthem. Systemic involvement is low.
Systemic involvement is low. Drugs with a high probability of reaction (3–5%): penicillin and related antibiotics, carbamazepine, allopurinol, gold salts (10–20%). Medium probability, sulfonamides (bacteriostatic, antidiabetic, diuretic), nonsteroidal anti-inflammatory drugs (NSAIDs), hydantoin derivatives, isoniazid, chloramphenicol, erythromycin, streptomycin. Low probability (<1%): barbiturates, benzodiazepines, phenothiazines, and tetracyclines.
Drugs with a high probability of reaction (3–5%): penicillin and related antibiotics, carbamazepine, allopurinol, gold salts (10–20%). Medium probability, sulfonamides (bacteriostatic, antidiabetic, diuretic), nonsteroidal anti-inflammatory drugs (NSAIDs), hydantoin derivatives, isoniazid, chloramphenicol, erythromycin, streptomycin. Low probability (<1%): barbiturates, benzodiazepines, phenothiazines, and tetracyclines. Exact mechanism unknown. Probably delayed hypersensitivity.
Exact mechanism unknown. Probably delayed hypersensitivity. Prior Drug Sensitization. Patients with prior history of exanthematous drug eruption will most likely develop a similar reaction if rechallenged with same drug.
Prior Drug Sensitization. Patients with prior history of exanthematous drug eruption will most likely develop a similar reaction if rechallenged with same drug. Sensitization occurs during administration or after completing course of drug; peak incidence at ninth day after administration. However, EDR may occur at any time between the first day and 3 weeks after the beginning of treatment. Reaction to penicillin can begin ≥2 weeks after drug is discontinued. In previously sensitized patient, eruption starts within 2 or 3 days after readministration of drug.
Sensitization occurs during administration or after completing course of drug; peak incidence at ninth day after administration. However, EDR may occur at any time between the first day and 3 weeks after the beginning of treatment. Reaction to penicillin can begin ≥2 weeks after drug is discontinued. In previously sensitized patient, eruption starts within 2 or 3 days after readministration of drug. Usually quite pruritic. Painful skin lesions suggest development of a more serious ACDR, such as toxic epidermal necrolysis (TEN).
Usually quite pruritic. Painful skin lesions suggest development of a more serious ACDR, such as toxic epidermal necrolysis (TEN). Systems Review. ± Fever, chills.
Systems Review. ± Fever, chills. Skin Lesions. Macules and/or papules, a few millimeters to 1 cm in size (
Skin Lesions. Macules and/or papules, a few millimeters to 1 cm in size ( Distribution. Symmetric (
Distribution. Symmetric ( Mucous Membranes. Enanthem on buccal mucosa.
Mucous Membranes. Enanthem on buccal mucosa. Laboratory. Peripheral eosinophilia. Dermatopathology: Perivascular lymphocytes and eosinophils.
Laboratory. Peripheral eosinophilia. Dermatopathology: Perivascular lymphocytes and eosinophils. Differential diagnosis includes all exanthematous eruptions: Viral exanthem, secondary syphilis, atypical pityriasis rosea, and early widespread allergic contact dermatitis.
Differential diagnosis includes all exanthematous eruptions: Viral exanthem, secondary syphilis, atypical pityriasis rosea, and early widespread allergic contact dermatitis. After discontinuation of drug, rash usually fades; however, it may worsen for a few days. The eruption may also begin after the drug has been discontinued. Eruption usually recurs with rechallenge, although not always.
After discontinuation of drug, rash usually fades; however, it may worsen for a few days. The eruption may also begin after the drug has been discontinued. Eruption usually recurs with rechallenge, although not always. The definitive step in management is to identify the offending drug and discontinue it. Oral antihistamine to alleviate pruritus. Glucocorticoids. Potent Topical Preparation May help speed resolution of eruption. Oral or IV Provides symptomatic relief. If offending drug cannot be substituted or omitted, systemic glucocorticoids can be administered to treat the ACDR. Prevention. Patients must be aware of their specific drug hypersensitivity and that other drugs of the same class can cross-react. Wearing a medical alert bracelet is advised.
The definitive step in management is to identify the offending drug and discontinue it. Oral antihistamine to alleviate pruritus. Glucocorticoids. Potent Topical Preparation May help speed resolution of eruption. Oral or IV Provides symptomatic relief. If offending drug cannot be substituted or omitted, systemic glucocorticoids can be administered to treat the ACDR. Prevention. Patients must be aware of their specific drug hypersensitivity and that other drugs of the same class can cross-react. Wearing a medical alert bracelet is advised. ICD-10: T88.7
ICD-10: T88.7 
 Acute generalized exanthematous pustulosis (AGEP) is an acute febrile eruption that is often associated with leukocytosis (
Acute generalized exanthematous pustulosis (AGEP) is an acute febrile eruption that is often associated with leukocytosis ( The estimated incidence is approximately 1–5 cases per million per year.
The estimated incidence is approximately 1–5 cases per million per year. Onset is acute, most often following drug intake, but viral infections can also trigger the disease.
Onset is acute, most often following drug intake, but viral infections can also trigger the disease. AGEP typically presents with nonfollicular sterile pustules occurring on a diffuse, edematous erythema (
AGEP typically presents with nonfollicular sterile pustules occurring on a diffuse, edematous erythema ( May be irregularly dispersed (
May be irregularly dispersed ( Fever and elevated blood neutrophils are common.
Fever and elevated blood neutrophils are common. Histopathology typically shows spongiform subcorneal and/or intraepidermal pustules; a marked edema of the papillary dermis; and eventually vasculitis, eosinophils, and/or focal necrosis of keratinocytes.
Histopathology typically shows spongiform subcorneal and/or intraepidermal pustules; a marked edema of the papillary dermis; and eventually vasculitis, eosinophils, and/or focal necrosis of keratinocytes. Pustules resolve spontaneously in <15 days and generalized desquamation occurs approximately 2 weeks later.
Pustules resolve spontaneously in <15 days and generalized desquamation occurs approximately 2 weeks later. Differential diagnosis includes pustular psoriasis, the hypersensitivity syndrome reaction with pustulation, subcorneal pustular dermatosis (Sneddon–Wilkinson disease), and pustular vasculitis.
Differential diagnosis includes pustular psoriasis, the hypersensitivity syndrome reaction with pustulation, subcorneal pustular dermatosis (Sneddon–Wilkinson disease), and pustular vasculitis. Acneiform pustular eruptions (see
Acneiform pustular eruptions (see 








