2 Origins and Psychiatric Management of Compulsive Eating Behaviors
Abstract
Obesity and weight loss are associated with unique neurochemical and physiologic changes that may have profound psychological implications. Obesity itself is frequently the result of an addictive overeating behavior pattern. Psychiatric disorders, including substance abuse and mood disorders, are common comorbidities in this subset of patients and cannot be ignored. A combination of psychotherapy, particularly cognitive-behavioral therapy and interpersonal therapy, and psychopharmacologic agents can help address these problems and improve the patient’s overall quality of life after weight loss and plastic surgery. It is important for the weight-loss physician and surgeon to recognize risk factors for psychiatric disease and be prepared to refer patients for appropriate evaluation and management both before and after bariatric and body-contouring surgery.
Introduction
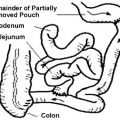
There is a worldwide epidemic of obesity, and there are many challenges that face the overweight individual struggling to achieve and sustain meaningful weight loss. In the developed world, despite the increasingly well-publicized health risks of obesity and the societal pressure to be thin, many people gain excess weight from childhood and throughout adult life. Over 50% of Americans are estimated to be obese or overweight,1 and the rate of recidivism with efforts at dieting and exercise remains high. Bariatric surgery has been a truly lifesaving and life-changing procedure and is the only treatment that has been found effective for long-term weight loss in the morbidly obese. But studies have shown that up to 20% of patients fail to achieve an acceptable weight loss or cannot sustain their weight loss beyond 2 to 3 years after surgery. This failure rate may actually increase with time after surgery, particularly in the most obese population, those with a body mass index (BMI) >50 kilograms per height in meters squared (kg/m2). These patients may slowly return to or surpass their preoperative weight, most often because of continued intake of foods with a high caloric density and failure to adopt a healthier lifestyle in terms of diet and exercise.2 How could a patient make the difficult decision to undergo surgery, perhaps even multiple surgeries, lose sufficient weight to have the experience of living in a more comfortable, more manageable, healthier body, and yet fail to follow through in a way that would sustain that progress?
Although it has been estimated from twin studies and studies in specific populations that as much as 70% of the variance in BMI is attributable to genetic factors and only 30% to the environment, the lesson learned from other epidemics is that societal and environmental causes also need to be addressed to bring about meaningful change.3 Although there are clearly multiple physiologic and psychological determinants of weight, the ultimate determinants are caloric intake and energy expenditure. Recent studies suggest that sensory preferences for energy-dense foods, usually those high in sugars and fats, are innate and present at birth. Given a choice, people tend to consume a set volume of food rather than a set volume of energy. Energy-dilute foods, such as vegetables, are more filling and satiating than low-volume sugars and fats, leading to overeating of these energy-dense foods. These preferences appear to have evolved to protect populations subject to periods of inadequate nutrition; animal studies show some of the same preferences for energy-dense foods.4
Thus, in the food-rich environment we now enjoy, with ample choices of quantity and types of food, perhaps the surprise is that obesity does not affect all of us, rather than that so many of us have difficulty overriding a behavior that gave our ancestors an evolutionary advantage. Some authors even describe obesity as perhaps an adaptive response to the current conditions of plenty in evolutionary terms, rather than an abnormality or disease. However, for some individuals, eating behaviors may take on lives of their own, persisting in the face of disability and even impending death.
Overeating as a Compulsive Behavior
Addictive behaviors present a challenge, as it is difficult for an observer to understand why clearly harmful behaviors are continued. However, we frequently see in practice alcoholics who drink despite liver damage or social consequences, smokers who smoke despite lung or heart damage, and morbidly obese patients who continue to overeat despite the resulting health risks and compromised function. In recent years, we have learned a great deal about the reinforcing effects of addictive substances on the brain. Neurochemical pathways involving such potent neurotransmitters as dopa-mine, serotonin, gamma-aminobutyric acid, and glutamate are affected by exposure to most substances with the potential for addiction or abuse. Repeated exposure to these addicting substances leads to stimulation and modulation of reward pathways that, in turn, make such substances more attractive and harder to resist. There is some evidence from neuroimaging that, even after years of abstinence, these pathways may never return to their preaddiction state, raising the possibility of relapse with reexposure.
Overeating clearly shares factors with other addictive behaviors. The overeater persists even in the face of negative physical consequences and societal disapproval, has a high frequency of relapse, and clearly finds rewards in the behavior that are not readily apparent to the observer and that override the obvious negative consequences. Recent studies in human and animal subjects suggest that some of the same neurotransmitter systems active in addiction may be involved in the persistence of harmful eating behaviors. Nora Volkow, one of the nation’s premier researchers on addictive behaviors, has proposed a common model for both obesity and drug abuse and addiction, with dopamine pathways in the brain playing a key role. In all addictive behaviors, she hypothesizes that the addicted individual assigns an enhanced salience or meaning to a specific reinforcer (drugs or food) at the expense of other reinforcers. This is due to conditioned learning and resetting of the brain’s reward thresholds by repeated stimulation either by substances of abuse or by large quantities of palatable food, usually nutrient-dense sugars or fats. In this model, exposure to the food or drug reinforcer or conditioned cues for consumption triggers the dopamine-modulated neuronal reward and motivation circuits, while inhibiting cognitive control.5 Thus, the drive to consume is stimulated, and the ability to inhibit the drive is diminished, a challenging combination to overcome even with the best of intentions. In a study looking at gender differences in brain response to food stimulation, Volkow and her colleagues6 found male subjects had a greater ability to inhibit brain activation in response to food stimulation in multiple regions, including the amygdala, hippocampus, insula, orbitofrontal cortex, and striatum. Volkow et al hypothesized that this difference in ability to dampen response to food stimulation may lead to a lower ability to suppress hunger in women and the resulting gender difference in obesity. Alternation of access and restriction may enhance the addictive eating pattern, leading to the familiar cycle of successful dieting followed by a rebound to the pre-diet weight or even higher.7 Imaging studies of obese and lean individuals who are anticipating calorie-rich tasty food versus a tasteless solution demonstrate that obese subjects respond with greater activity in the gustatory and somatosensory regions of the brain and decreased activation in the striatum. This decreased activity may reflect a genetically mediated decrease in dopamine signaling in that region, leading these individuals to overeat to compensate for the hypofunctional dorsal striatum.8,9
The continued growth of obesity in our society despite the proliferation of “lite” and “low-fat” foods emphasizes that there is no shortcut to healthy eating, and a recent commentary in the Journal of the American Medical Association points out that even efforts to curb calorie intake through the use of artificial sweeteners may not trump neurobiology. The author cites an observational study that found a dose–response relationship between consumption of diet drinks and measurement of adiposity over time, as well as studies finding daily consumption of such drinks associated with metabolic syndrome and risk of diabetes, although the direction of the causality was not clear. Even more concerning, studies with experimental animals found the drive for sweetness led them to choose saccharine over intravenous cocaine, previously thought to be one of the most addictive substances. The risk for the human would-be dieter is that the use of these hyper-sweet sugar substitutes may over-stimulate sweetness receptors, causing them to revert to an infantile state and making a healthy diet of energy-dilute foods less palatable.10 David Kessler,11 the former chief of the Food and Drug Administration who was widely praised for addressing tobacco addiction during his term in office, has joined the struggle against obesity. In a recent book, he calls for retraining our brains, suggesting that we must make the same change in societal attitudes toward the acceptability of eating calorie-rich foods that we did toward the acceptability of smoking. He challenges our society to trade the transient neurologic reward of high-sugar/high-fat foods for healthier eating, while acknowledging his own lifelong struggle to resist those very foods. However intellectually appealing his arguments may be, experience, including his own, suggests that these behavioral changes are difficult to maintain long-term, and the data from neuroimaging studies confirm that a formerly obese individual’s neurobiology may be producing a silent but very powerful opposition to such change. For most would-be dieters, an ice cream soda will continue to look more appealing than brussels sprouts, and their own neurotransmitters will continue to urge them in the direction of the fats and sugars that will bring satiation and neurologic reward. A recent article in the New Yorker re-viewed some of the societal factors that also militate against dietary behavior change, including a decline in the cost of fats, oils, and sugars relative to other foods, and the successful commercial promotion of larger servings extending even into the home, as newer editions of old standby cookbooks now calculate fewer servings from standard recipes.12
Psychiatric Disorders Associated with Obesity
Just as behaviors involving use of other substances warrant their own psychiatric diagnoses as disorders of dependence or abuse, some eating patterns warrant specific diagnoses as eating disorders, involving restriction, excess, or both. Volkow and O’Brien13 have proposed that the forthcoming fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), anticipated to be released in 2012, should recognize some forms of obesity as mental disorders of addiction and dependence, citing the many physiologic and behavioral overlaps with the other addictive disorders. Yet eating clearly differs from the other disorders of abuse, as we all must eat to live. Eating is a basic biologic drive, and abstinence is an impossible goal. Thus, the study of overeating and morbid obesity must focus on a very complex behavior that is influenced by a wide range of biopsychosocial factors, from genetics, to brain chemistry, to family pressures, to self-image.
All obese patients overeat, but not all overeating is the same. Individuals diagnosed with binge-eating disorder (BED) have more severe obesity, earlier onset of overweight and dieting, and more comorbidity with both other psychiatric disorders and substance abuse. Binge eating is defined as overeating for discrete periods of time, marked by a sense of loss of control. Periods of binging may last for an entire day, and the binging may be superimposed on a background of overeating.14 A study that compared dopamine and opioid receptors in binging and nonbinging obese adults suggests that BED may be biologically based and driven by a heightened response to the pleasurable properties of food.15 Binging is sometimes linked to purging behaviors, which tend toward weight neutrality or even anorexia in patients with binging, purging, and restriction, or bulimia nervosa. Episodes may have no clearly demarcated beginning or end and may last for an entire day. Although the prevalence of binge eating among the obese population is debated, with estimates ranging from 1 to 30%, it does appear to be more prevalent in women, and identification of this eating pattern is important in treatment considerations, as it may be linked to more severe obesity, earlier onset of both obesity and dieting, and more severe psychopathology.14
Another, more recently identified, eating disorder is night-eating, a triad of morning anorexia, evening hyper-phagia, and insomnia.16 This pattern may be accompanied by depression, which also follows a circadian pattern, with symptoms more prominent during evening and nighttime hours. Problematic night-eating is seen more frequently in the obese and during periods of stress and may remit when the stress is alleviated. It is rare in individuals of normal weight but has been reported in almost 9% of those attending an obesity clinic, 27% of those in surgical weight-loss programs, and 5% of those presenting for treatment of insomnia.14,16 Such individuals take in more than 50% of their daily caloric intake between 10 p.m. and 6 a.m., compared with obese control subjects who consume 15% of their calories during that time frame. The night-eaters have more disrupted sleep and awaken more than three times as often as controls, with almost 50% of these awakenings leading to food intake. Confirming the association with stress, cortisol levels are higher among the night-eaters.14
It is interesting to consider the links between eating behaviors and some of the major Axis I psychiatric disorders. Although metabolic disorders, including weight gain, can be seen with the use of antipsychotic medications for schizophrenia or bipolar disorder and antidepressants for depression, these disorders themselves may have an association with weight gain or inappropriate eating behaviors. Increased obesity and visceral adiposity have been found in some samples of medication-naive schizophrenics,17 whereas the behavior dysregulation and impulsivity seen in manic bipolar patients can lead to overdoing anything, including food. Although patients with major depression typically lose appetite and weight, there are atypical depressions marked by overeating and weight gain. Patients with anxiety disorders may eat to relieve stress in general or in particularly stressful situations. As these major psychiatric disorders are the end result of dysfunction in the regulation of brain chemistry, it is not surprising that overeating, with its powerful effects on neurotransmitters, would be a final common pathway for self-soothing or even self-medication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree