Cutaneous Lymphomas in HIV-Infected Individuals
Patients infected with the human immunodeficiency virus (HIV) may develop benign and malignant lymphoproliferative disorders [1–3]. It has been estimated that HIV+ patients have a risk of developing a malignant lymphoma up to 200-fold higher than normal individuals. The onset of lymphoproliferative disorders has been observed particularly in individuals with overt acquired immunodeficiency syndrome (AIDS) [4–6]. There are overlapping features between the lymphomas arising in HIV+ patients and those observed after solid organ or bone marrow transplantation, but also some aspects peculiar to HIV+ individuals, and HIV-related lymphomas are included as a specific category in the World Health Organization (WHO) Classification of Tumours of Hematopoietic and Lymphoid Tissues [7]. Most lymphoproliferative disorders that arise in HIV+ patients occur at sites other than the skin, but primary cutaneous cases or secondary cutaneous involvement from extracutaneous lymphomas may be observed. Two types of lymphoma frequent in HIV+ individuals, namely, primary effusion lymphoma and plasmablastic lymphoma, are only rarely observed in the skin in these patients (these two lymphomas are discussed in Chapter 15).
Most cutaneous lymphomas in HIV+ patients represent examples of “conventional” skin lymphomas such as mycosis fungoides, anaplastic large cell lymphoma, or diffuse large B-cell lymphoma, leg type, similar to what can be observed in cutaneous lymphomas arising after solid organ transplantation (see Chapter 15). In contrast to cutaneous lymphomas in the general population, however, mycosis fungoides represents a minority of the cases arising in HIV+ patients, and B-cell lymphomas are more common. An exceptional case of primary cutaneous Burkitt lymphoma in an HIV+ individual has also been described [8].
Association with Epstein–Barr virus (EBV) has been documented in most cases of plasmablastic lymphoma and in other cutaneous B-cell lymphomas in HIV+ individuals, and rarely also in cases of cutaneous anaplastic large cell lymphoma associated with HIV. Cases of HIV-related cutaneous anaplastic large cell lymphoma positive for human herpes virus-8 (HHV-8) have been documented as well [9], and infection with human T-lymphotropic virus 2 (HTLV-II) has been detected in one HIV+ patient with a CD8+ cutaneous T-cell lymphoma [10].
Clinical Features
Patients are adults who are infected with HIV and have a very low CD4 count, but rarely children can be affected as well [4, 11]. The onset of a cutaneous lymphoma may represent the first AIDS-defining illness in some of these patients. Cutaneous lesions are variable, depending on the type of lymphoma, and are not different from those observed in the corresponding lymphoma types arising in non-HIV-infected individuals. Accurate staging investigations should be performed in order to evaluate the extent (if any) of extracutaneous involvement.
In patients with HIV infection, mycosis fungoides should be distinguished from benign CD8+ cutaneous infiltrates, which may reveal similar clinicopathologic features (see Chapter 26).
Plasmablastic lymphoma presents mostly with nodules and tumors arising in the oral mucosa (or at other mucosal sites), but cutaneous involvement has been reported rarely (see Chapter 15) [12].
Histopathology, Immunophenotype, and Molecular Genetics
Histopathologic, immunophenotypical, and molecular genetic features are similar to those observed in the corresponding lymphoma entities arising in non-HIV-infected individuals. However, in cases of diffuse large B-cell lymphoma, plasmablastic differentiation and association with EBV are more common. Rarely, a post-transplant lymphoproliferative disorder-like polymorphic infiltrate may be observed (see Chapter 16) [13]. In early lesions plasma cells are usually polyclonal (Fig. 17.1). Staining for infectious organisms, particularly syphilis, are crucial in such cases, as cutaneous infections are common in HIV+ patients and may present with atypical lymphoid infiltrates resembling lymphoma (see Chapter 26).
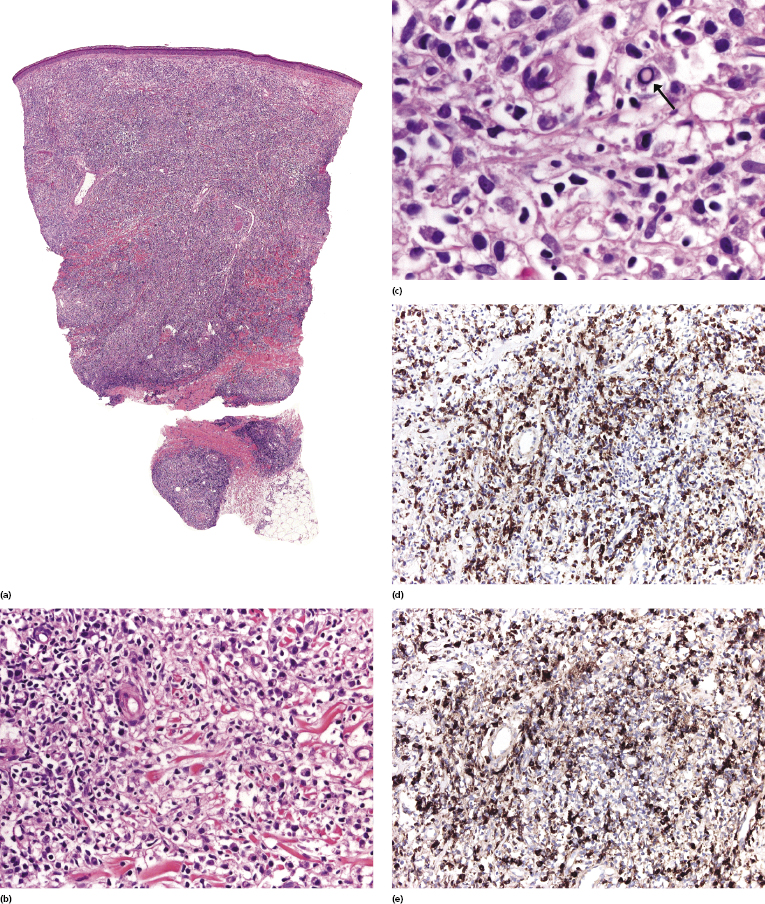
HIV-related cutaneous anaplastic large cell lymphoma presents with histopathologic and immunophenotypic features similar to those observed in the non-HIV-related type. Different morphologic subtypes may be observed [14, 15]. ALK-1 is not expressed by neoplastic cells. EBV and HHV-8 are usually negative, but may be detected in a minority of cases.
In some cases, HIV-related cutaneous lymphomas cannot be classified into a precise category and may show overlapping features between different lymphoma types, a situation similar to what can be observed in other immunodeficiency-related cutaneous lymphomas. Of note, B-cell lymphomas associated with HHV-8 in HIV+ patients may show an anaplastic morphology similar to that of anaplastic large cell lymphoma and express CD30, thus representing a potential pitfall [16, 17].
Treatment and Prognosis
As these lymphomas arise in patients who are profoundly immunocompromised, death often ensues, resulting from complications of AIDS rather than from direct lymphoma spread. Therapy should be directed at both the HIV disease and the lymphoma [1]. Conservative treatment strategies have been advocated for cutaneous lymphomas [4]. In this context, it has been shown that after the introduction of highly active antiretroviral treatment (HAART) the frequency of the more aggressive types of HIV-associated lymphomas decreased and the outcome has improved [18].
Mycosis fungoides in HIV+ patients should be managed according to standard protocols (see Chapter 2) [4, 19]. Solitary or localized lesions of other lymphomas may be treated with local radiotherapy. If not already introduced, HAART should be started.
HIV-associated cutaneous anaplastic large cell lymphoma seems to have a worse prognosis than the non-HIV-related variant [20], thus conservative strategies may fail in this particular setting.
Prognostic features for HIV-associated T-cell lymphomas have been studied for non-cutaneous cases [21]. Use of HAART and the patient’s performance status were associated with overall survival in multivariate analysis [21].

Full access? Get Clinical Tree


