Cutaneous Post-transplant Lymphoproliferative Disorders
Lymphoproliferative disorders are one of the most common malignancies in recipients of solid organ and bone marrow transplantation, developing in approximately 2% of patients. They are listed in a specific chapter in the World Health Organization (WHO) Classification of Tumours of Hematopoietic and Lymphoid Tissues [1]. For the most part they represent examples of Epstein–Barr virus (EBV)-associated lymphoproliferative disorders. Although cutaneous manifestations are rare, some patients may present with disease localized solely to the skin [2–8]. Many cases arise within the first year after organ or bone marrow transplantation, but the time interval between transplantation and the onset of a post-transplant lymphoproliferative disorder may be much longer.
In general, post-transplant lymphoproliferative disorders occur more often in recipients of heart–lung allografts and less commonly in those who receive renal allografts [1]. However, in a large European study on cutaneous cases, the majority of patients had a renal transplant [9]. Other risk factors include primary infection with EBV following transplantation, infection with cytomegalovirus, T-cell-specific immunosuppression, and younger age [10]. At extracutaneous sites, there is a higher risk of developing hepatosplenic T-cell lymphoma, Burkitt’s lymphoma, natural killer (NK)/T-cell lymphoma, diffuse large B-cell lymphoma, and anaplastic large-cell lymphoma [11]. In the skin, on the other hand, about half of the cases are represented by mycosis fungoides [9]. Interestingly, in B-cell post-transplant lymphoproliferative disorders clonal populations of T lymphocytes can also be detected, suggesting that a lack of immune surveillance may affect the T- as well as the B-cells [12].
Post-transplant lymphoproliferative disorders are classified according to four major categories [1]:
- Early lesions (reactive plasmacytic hyperplasia, infectious mononucleosis-like lesions).
- Polymorphic post-transplant lymphoproliferative disorder.
- Monomorphic post-transplant lymphoproliferative disorder, with clinicopathologic features corresponding to “conventional” entities of B- and NK/T-cell lymphomas.
- Classical Hodgkin lymphoma-type post-transplant lymphoproliferative disorder.
At extracutaneous sites, most monomorphic lesions exhibit a B-cell phenotype, but in rare instances an NK/T-cell phenotype has been observed. The occurrence of NK/T-cell lymphomas, and particularly of cutaneous anaplastic large cell lymphoma, seems to be more frequent in the skin than in other organs [9, 13]. As already mentioned, mycosis fungoides is the most frequent cutaneous lymphoma in the post-transplant setting [9, 14], and one case has been observed also in a pediatric patient as part of a post-transplant cutaneous composite lymphoma [15].
Besides conventional post-transplant lymphoproliferative disorders, the onset of adult T-cell lymphoma/leukemia (ATLL) has been documented in three patients who received organs from a patient infected with human T lymphotropic virus 1 (HTLV-1) [16].
Clinical Features
Patients are adults or children under immunosuppressive treatment after allogeneic solid organ or bone marrow transplantation. Cutaneous lesions are variable, including erythematous plaques, nodules, and tumors, sometimes ulcerated (Figs 16.1 and 16.2). They can be solitary, localized to a single anatomic region or generalized. Concomitant involvement of other organs can be observed and is usually associated with systemic symptoms, including elevated serum levels of lactic dehydrogenase (LDH). Precise staging investigations should always be performed, in order to evaluate the extent of involvement before planning the treatment.
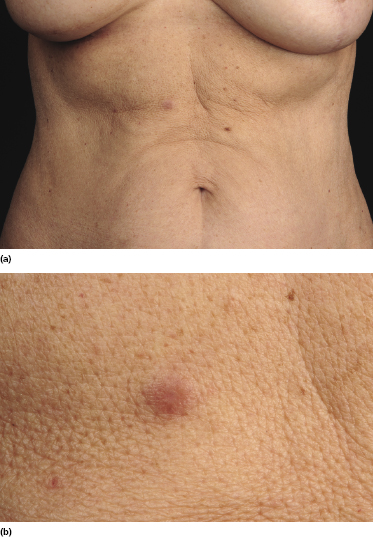
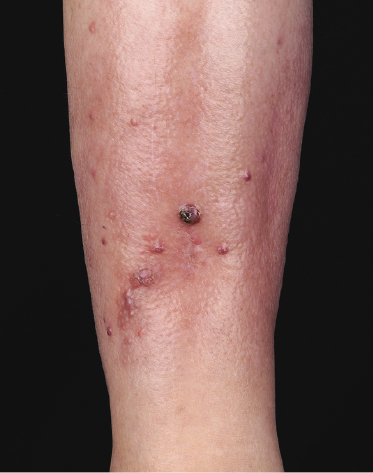
Histopathology, Immunophenotype, and Molecular Genetics
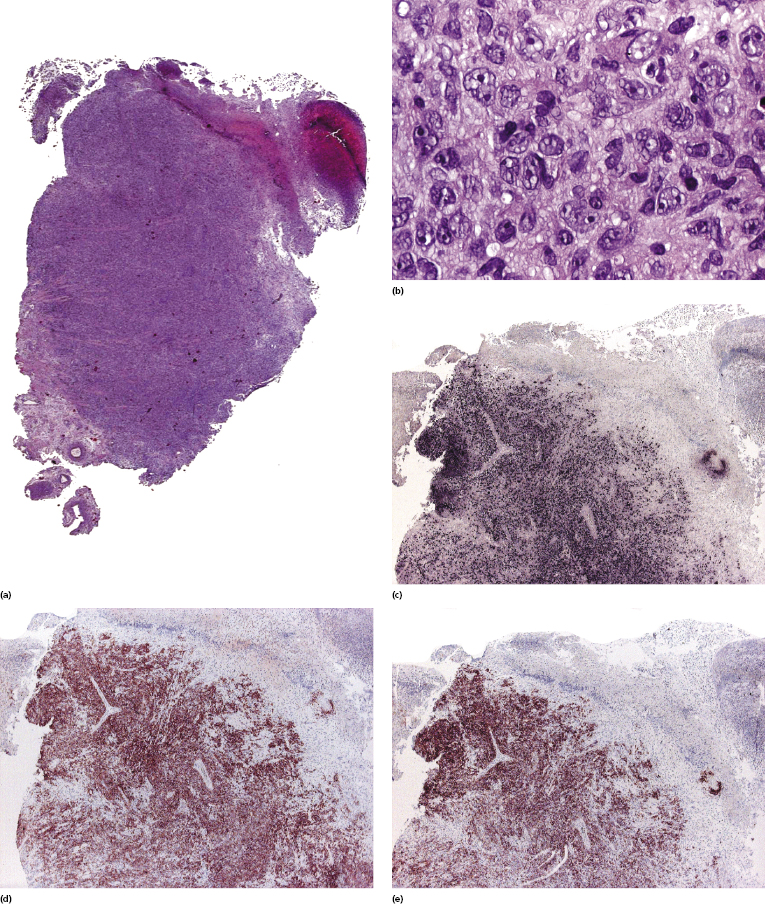
Histopathologic features vary according to the subtype of post-transplant lymphoproliferative disorder. Early lesions show polyclonal (rarely monoclonal) proliferations of mature plasma cells with rare immunoblasts. Polymorphic post-transplant lymphoproliferative disorder is characterized by the presence of a monoclonal infiltrate of B lymphocytes, comprising the whole spectrum of maturation and including plasma cells, immunoblasts, and intermediate-sized lymphoid cells (Figs 16.3 and 16.4).
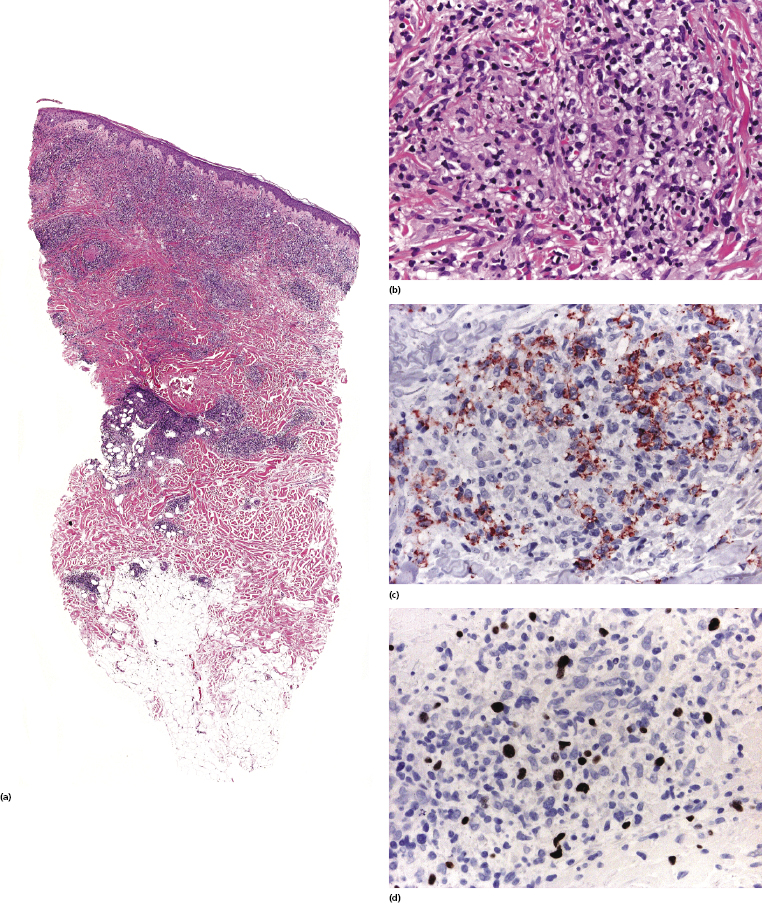
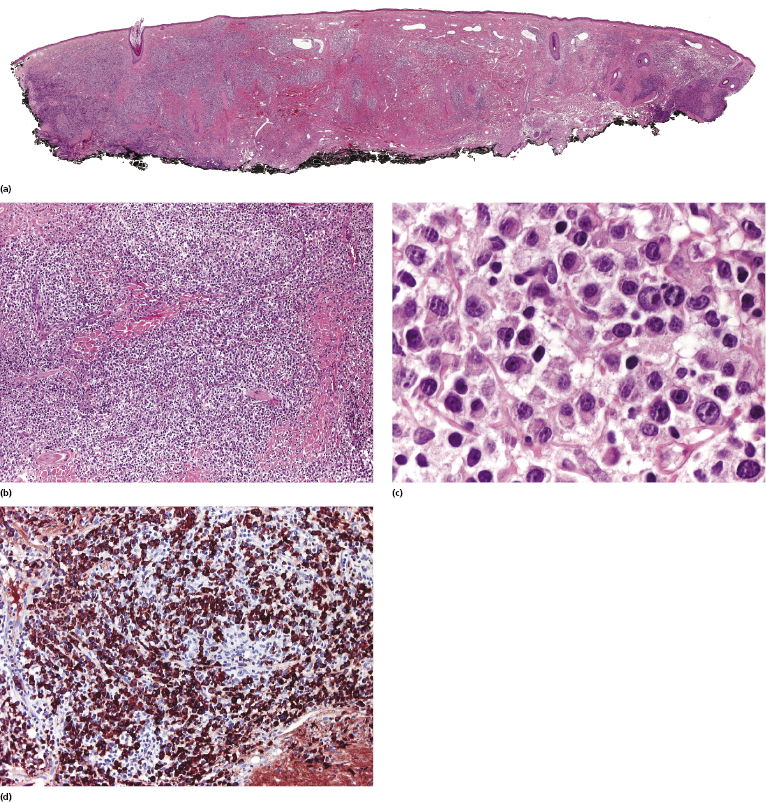
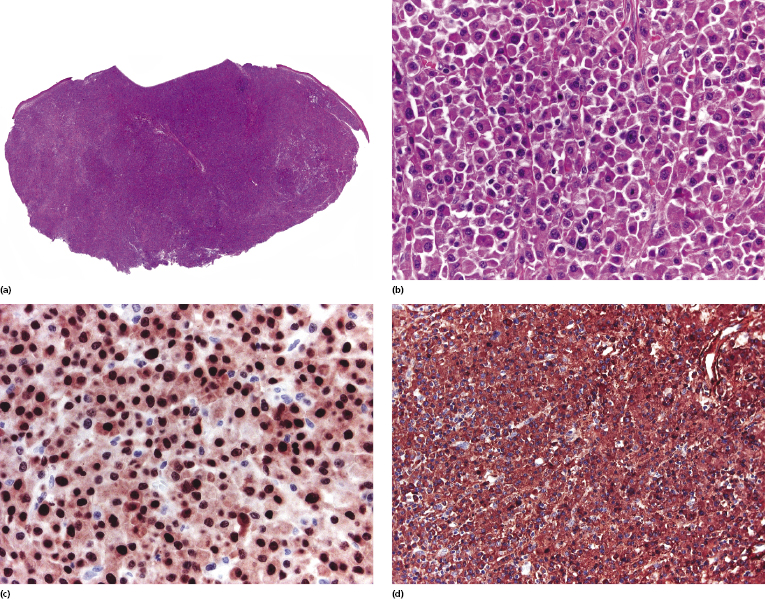
In monomorphic post-transplant lymphoproliferative disorder arising at extracutaneous sites, the histopathologic picture is more frequently that of a B-cell lymphoma (diffuse large B-cell lymphoma, Burkitt lymphoma, plasma cell myeloma, or extramedullary plasmacytoma). Analysis of phenotypic and molecular features of a large group of cases confirmed the diversity of different cases of monomorphic post-transplant lymphoproliferative disorders [17]. CD30 is frequently expressed. Post-transplant monomorphic B-cell lymphomas involving the skin present often with the features of diffuse large B-cell lymphoma or plasmablastic lymphoma (Fig. 16.5) [18, 19]. Rare cases are morphologically indistinguishable from plasmacytoma, with immunoglobulin light chain restriction and MUM-1 expression (Fig. 16.6) [20–23]. Post-transplant plasmacytomas are not always associated with EBV, usually arise several years after transplantation, and seem to have a better prognosis when compared to other post-transplant lymphomas [21]. One case resembling lymphomatoid granulomatosis has been described as well [24].
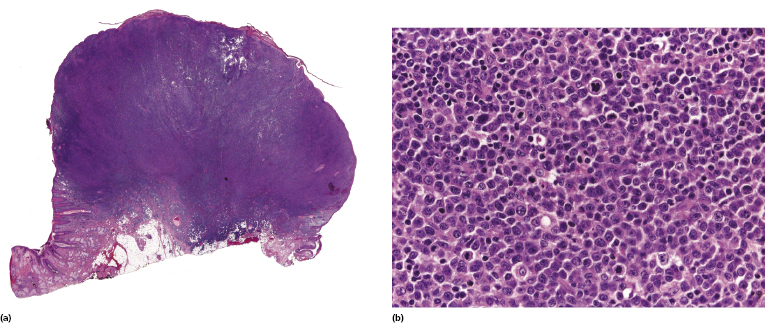
As already mentioned, in the skin the occurrence of post-transplant lymphoproliferative T-cell disorders, particularly mycosis fungoides and cutaneous anaplastic large cell lymphoma, is more common than at extracutaneous sites (Fig. 16.7) [9, 13, 25–29]. These cases represent approximately half of all cutaneous post-transplant lymphomas, again showing the general higher frequency of T-cell lymphomas in the skin as opposed to the lymph nodes. A case of subcutaneous panniculitis-like T-cell lymphoma has been observed in a cardiac allograft recipient [30]. Although cutaneous T-cell lymphomas in immunocompromised patients are usually not associated with EBV infection, EBV+ cases can be observed rarely [31].
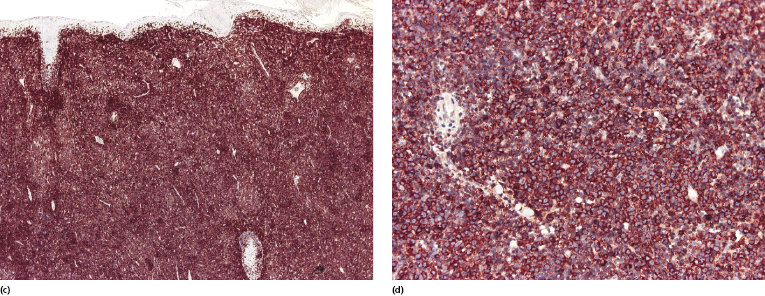
Genetic analyses showed that gains of chromosomes 5p and 11p and deletions of chromosome 12p were common in the diffuse large B-cell lymphoma variant of post-transplant lymphoproliferative disorders, and that some overlapping features between diffuse large B-cell lymphomas in immunocompetent and immunosuppressed individuals could be observed [32]. High levels of hypermethylation of the tumor suppressor gene p16 have been demonstrated in one EBV-associated cutaneous case, supporting the evidence for a carcinogenic role of EBV in positive cases [33].
Treatment and Prognosis
Reduction of immunosuppression, often associated with antiviral therapy, is the main strategy for treatment of post-transplant lymphoproliferative disorders [34–36]. Regression of the lesions may take place over a period of a few weeks up to a few months. Lesions that do not respond to these measures may be treated by more conventional modalities, including radiotherapy, interferon-α, anti-CD20 antibody (rituximab) (only for cases with B-cell phenotype), and systemic chemotherapy.
Plasmacytic hyperplasia and probably also plasmacytoma-like tumors almost always respond completely to reduction of immunosuppression [21]. The prognosis of polymorphic or monomorphic post-transplant lymphoproliferative disorders limited to the skin seems to be more favorable than that of extracutaneous post-transplant lymphoproliferative disorders. Although some cases of post-transplant cutaneous anaplastic large cell lymphoma had a favorable behavior [37, 38], in general mycosis fungoides and anaplastic large cell lymphoma in immunosuppressed individuals do not share the same favorable prognosis as in immunocompetent hosts [9, 13, 14, 39].

Full access? Get Clinical Tree


