14 Complications of Velopharyngeal Insufficiency Surgery and Special Populations
Introduction
Velopharyngeal insufficiency (VPI) is incomplete closure of the velopharynx characterized by hypernasal resonance, inappropriate nasal emission during phonation, and nasal regurgitation. The common causes of VPI in children are overt or submucous cleft palate, insufficiency following adenoidectomy, and, less frequently, congenital and acquired neuromuscular disorders.
The management of VPI involves a multidisciplinary team of specialists, including a speech-language pathologist and surgeon, and frequently requires a combination of surgical and nonsurgical approaches. Nonsurgical options include speech therapy and prosthetic management. Surgical treatment can involve augmentation with injectable or implantable materials, palatal lengthening or repositioning procedures, and, more commonly, pharyngeal flap (PF) or sphincter pharyngoplasty (SP). Both PF and SP have high success rates for treatment of VPI; however, complications have been well described in the literature. The goal of eliminating hypernasal resonance while avoiding nasal obstruction represents a major challenge in the surgical treatment of VPI. This chapter describes the prevention and management of complications after VPI surgery.
Perioperative Management
Overview
The reported risk for complications after the surgical treatment of VPI varies. Early reports of high complication rates raised concerns about the safety of VPI surgery.1–3 In an effort to improve the safety of surgical VPI treatment, Fraulin et al.4 looked at acute perioperative complications after PF procedures. The authors identified factors that were predictive of overall increased complication rate, which included associated medical comorbidities and technical features of the procedure (e.g., combined procedures at the time of PF or failure to close the pharyngeal donor site). They also identified age as a possible risk factor for postoperative obstruction, noting that patients who developed airway obstruction were on average younger than those without any airway compromise. The one death in this series occurred after respiratory failure in a patient with a neuromuscular disorder. In addition, Witt et al.5 focused on the development of obstructive sleep symptoms after SP and suggested the following factors contributed to airway compromise: documented micro- and retrognathia, history of perinatal respiratory dysfunction, early age at SP surgery, and concurrent upper respiratory tract infections.
Preoperative Management
Preoperative management of VPI should include a thorough workup and careful patient selection. Polysomnography is indicated for all high-risk patients, including those with Pierre Robin sequence, Treacher Collins syndrome, or Goldenhar syndrome, and those who report a history of snoring, apneic events, or other symptoms suggestive of obstructive sleep apnea (OSA).4,6,7 Preoperative OSA is considered to be a relative contraindication for most VPI procedures, particularly the obstructing ones (i.e., PF).6,7 Similarly, hypotonia is a relative contraindication for VPI surgery, and some authors recommend that nonsurgical management be considered in patients with a neuromuscular cause of VPI.6,8
Another preoperative consideration includes the treatment of adenotonsillar hypertrophy prior to proceeding with VPI repair. Tonsillar hypertrophy is associated with the development of OSA after PF. Many cleft centers advocate a preemptive tonsillectomy and selective adenoidectomy prior to secondary speech surgery. This is thought to reduce the risk of postoperative sleep-disordered breathing and OSA. Also, there is a theoretical benefit of limiting the variation of air escape depending on adenoid size during upper respiratory infections. In a large retrospective review of PF surgery, Ysunza et al.9 demonstrated that 87% (13/15) of patients who developed postoperative OSA had enlarged tonsils. Tonsillar hypertrophy and posterior displacement of tonsils can occur postoperatively and cause obstruction of the lateral ports. For this reason, many authors recommend that tonsillectomy and adenoidectomy be performed on all patients at least 8 weeks prior to PF.6,10 The protocol for preoperative tonsillectomy and adenoidectomy before SP is less detailed in the literature, and practice patterns of surgeons are variable. There is a theoretical risk of posttonsillectomy scarring resulting in the restricted movement of SP flaps; however, this has not been directly studied.
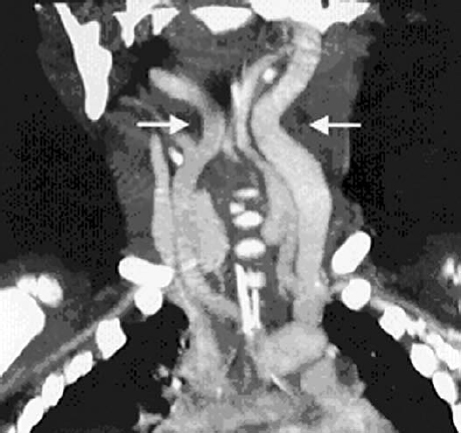
Although routine blood work is not indicated in otherwise healthy children, if there is concern for bleeding disorders or platelet dysfunction, appropriate laboratory work and correction of bleeding abnormalities should be completed prior to undergoing VPI surgery. Several studies cite younger age as a possible risk factor for perioperative complications. Fraulin et al. found that patients who developed airway obstruction after PF were younger than patients who did not develop this complication (7.8 years versus 9.3 years).4 In a retrospective review of SP, all cases of prolonged airway compromise occurred in patients 5 years old or younger.5 However, it is has been shown that SP can be performed safely in children as young as 3 years of age.11 Consideration should be given to assessing the position of the internal carotid arteries in patients with DiGeorge or velocardiofacial syndromes ( Fig. 14.1 ).
Intraoperative Management
Intraoperative modifications can also reduce complications. An open donor site after PF surgery was found to be a risk factor associated with an increased overall complication rate, specifically with increased risk of bleeding complications.4 Many authors advocate for closure of the donor site in PF and SP.4,6,10,12
There is some debate as to whether concurrent procedures increase the risk of airway swelling, and therefore increase perioperative complications. In a series of 386 patients, associated procedures including palatoplasty, palatal fistula repair, alveolar bone grafting, or maxillary osteotomies at the time of PF were found to be a risk factor for the development of any complication.4 Similarly, Wray et al.13 found higher rates of airway obstruction in patients who had concurrent palatoplasty and PF. In a more recent study, Milczuk et al.14 found that SP and Furlow palatoplasty could be safely combined as a single-stage procedure without any increased risk of airway complications.
Postoperative Management
Postoperative management of VPI surgery is critical, as most complications, including airway obstruction and bleeding, occur within the first 24 hours.4,15 Emergence from anesthesia without coughing or straining and avoiding deep laryngeal suction is ideal. The literature recommends different levels of postoperative care after PF and SP, including intensive care unit admission,4 step-down unit monitoring,6 pediatric surgical floor status,16 or outpatient surgery in the case of uncomplicated SP procedure.17 Nearly all articles advocate the use of continuous pulse oximetry monitoring in the initial postoperative setting. The use of nasopharyngeal airways anecdotally prevented airway obstruction in one series,4 and other authors describe the use of nasal stenting in the immediate postoperative period using endotracheal tubes or red rubber catheters for 1 to 2 days.8
Bleeding
Postoperative bleeding is one of the most common complications after VPI surgery. Early series of PF reported bleeding rates as high as 14%1; however, more recent studies show that bleeding occurs in 0 to 6% of patients after PF.4,6,8,10,11,15,16,18 Similar rates of bleeding, ranging from 0 to 4%, occurred after SP.11,14,17,18 The majority of cases occurred within the first 24 hours after surgery. Interventions included observation in patients with mild bleeding, transfusion of blood products, and surgical exploration for hemostasis.6,14,17,19 Generally, all but mild, self-resolving bleeding was taken back to the operating room for exploration. The majority of cases did not require transfusion of blood products, and there were no reported cases of flap takedown for acute, postoperative bleeding.
Airway Obstruction
Acute Airway Obstruction
Airway obstruction following VPI surgery is a well-recognized, potentially fatal complication that deserves special consideration. Airway compromise can be divided into acute complications, including postoperative nasal obstruction or oxygen desaturation, and long-term complications, including OSA, chronic nasal obstruction, and continued general respiratory complaints. Acute airway obstruction has been documented in up to 9% of patients after PF procedures but less so for SP,1 although the majority of studies report lower rates of respiratory compromise ranging from 0 to 3%.4,8,16,20 Most cases of perioperative obstruction after PF occur within the first 24 hours after surgery.4,8,15 Fraulin et al.4 found that cases of airway obstruction within the first 24 hours were more severe and required invasive management including reintubation or jaw thrust and ventilation. Patients who developed obstruction after 24 hours were treated conservatively with repositioning while sleeping and continuous monitoring. Other series documented only mild cases of airway obstruction in the first 24 hours. In these studies, patients with limited or transient oxygen desaturation responded to minor interventions, such as supplemental oxygenation through rebreather facemask, stimulation, or prone positioning.6,19 Acute airway obstruction resulting in death after PF is rare but has been reported in several studies.1,2,20
Chronic Airway Obstruction
In most cases, acute airway compromise following PF will resolve with conservative management; however, there is a subset of patients that develops chronic airway obstruction. The etiology of OSA is likely a combination of an anatomically reduced airway dimension, inflammation and edema, and transiently decreased pharyngeal muscle tone.15,21,22 Early studies documented a high incidence of OSA following PF.23 Results from more recent studies found postoperative OSA to be less common, ranging from 0 to 3%.6,10,16,20 General respiratory complaints, including snoring, mouth breathing, and other sleep obstructive events, are more common and have been documented in up to 89% of patients.24 In a prospective study of patients undergoing PF for VPI, 55% of patients reported respiratory complaints 5 months after surgery. One year following PF, 36% of patients continued to have respiratory symptoms.25
Although airway obstruction following PF is well documented, there is a lack of evidence demonstrating similar rates of obstruction following SP. Witt et al.5 reported a 13.8% rate of airway dysfunction after SP. Of note, five of the eight patients had Pierre Robin sequence, and only two patients continued to have airway compromise after postoperative day 3. Since that time, multiple series have documented low rates of OSA after SP, ranging from 0 to 4%, but this rate likely increases with revision surgery.7,12,17 In the past, it was generally accepted that PF had higher incidence of postoperative obstruction when compared to SP. This was supported by de Serres et al.26 who reported that OSA was confirmed in all PF patients with postoperative sleep symptoms, while none of the SP with sleep symptoms had OSA on polysomnography. Over the past decade, however, randomized, prospective studies have demonstrated no significant difference in the rate of OSA following PF and SP.11,18
Surgical modifications of PF and SP based on clinical evaluation may decrease the rate of postoperative OSA. Nasal endoscopy and/or videofluoroscopy are used for preoperative evaluation to determine the extent of lateral wall movement and the size of the velopharyngeal gap. Some authors suggest that the size of the PF should be tailored to the mobility of the lateral walls. In order to decrease the likelihood of postoperative obstruction, patients with good mobility of the lateral walls should have narrower flaps.27 Chegar et al.6 attributed their low incidence of breathing complications to the use of high, short flaps harvested above the soft palate and vertical advancement of the donor site to avoid pharyngeal narrowing. SP procedures are also customizable.
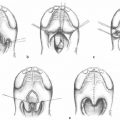
Sie et al.7 described a relationship between the patients who developed nasal obstruction after SP and small velopharyngeal gap on physical exam. The flap design was modified posterior and lateral to the tonsillar pillar to decrease the closure of the sphincter with flap transposition, and thereby reduce airway obstruction. De Serres et al.26 echoed the relationship between small velopharyngeal gaps and the development obstructive symptoms after SP. In this study, myomucosal flaps were modified such that the tonsillar pillars were not incorporated.26
Treatment of Airway Obstruction
There is not a well-accepted protocol for the treatment of OSA following VPI surgery. Polysomnography should be obtained in patients where there is a clinical suspicion for OSA; however, the timing of when to obtain the sleep study is not clearly defined. Sirois and colleagues28 found OSA in 15% of patients in the early postoperative period following PF; however, repeat sleep study in the following months revealed resolution of OSA. Based on these findings, it is reasonable to manage selected patients expectantly.15 Continuous positive airway pressure (CPAP) ventilation is an effective option for patients who decline surgery.5,10 Rarely, patients will need flap revision or removal due to OSA.16,20
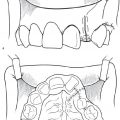
Por et al.29 retrospectively reviewed methods for relieving airway obstruction after PF, namely release and Z-plasty of the lateral ports and division of the PF with or without Furlow palatoplasty. Release of the lateral ports after PF has been previously described and may lead to recurrent airway obstruction due to scar contracture.30 Division of the PF flap has been reported to preserve velopharyngeal sufficiency in some cases; however, flap takedown can be complicated by reattachment and subsequent obstruction.31,32 Por et al.29 reported improved results when PF division was combined with Furlow palatoplasty. The authors proposed that the combined procedure preserved velopharyngeal sufficiency by repositioning palatal muscles and narrowing the pharyngeal walls. The addition of palatoplasty may also decrease the chance of reattachment by reorienting the incision away from the wound on the posterior pharyngeal wall. OSA from a narrow SP can be revised ( Fig. 14.2 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree