13 Sphincter Pharyngoplasty
Introduction
Sphincter pharyngoplasty is one of a handful of surgical procedures used to treat velopharyngeal dysfunction (VPD) in children. Nomenclature for VPD procedures is quite similar and may therefore be confusing. Surgical correction of VPD in children can be classified by the specific musculature of the velopharyngeal apparatus each procedure addresses. The most commonly described VPD procedures manipulate the palate (e.g., double-opposing Z-palatoplasty), the pharynx (sphincter pharyngoplasty), or both the palate and pharynx (posterior pharyngeal flap). In the Furlow double-opposing Z-palatoplasty, the levator veli palatini musculature is reoriented from its aberrant sagittal positioning to a more natural transverse orientation, while opposing oral and nasal flaps are transposed into a “z.” The resulting lengthening of the soft palate is particularly effective for children with overt or occult submucous cleft palate, as well as in children with previously repaired cleft palate with evidence of sagittal levator veli palatine musculature.1 In the posterior pharyngeal flap, a superiorly based myomucosal flap is elevated and inset into the central portion of the velum, thereby permanently obturating the velopharynx and allowing nasal respiration via two lateral ports. Many surgeons continue to use the superiorly based posterior pharyngeal flap as their workhorse procedure for management of VPD.
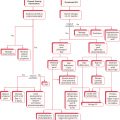
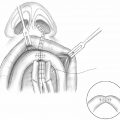
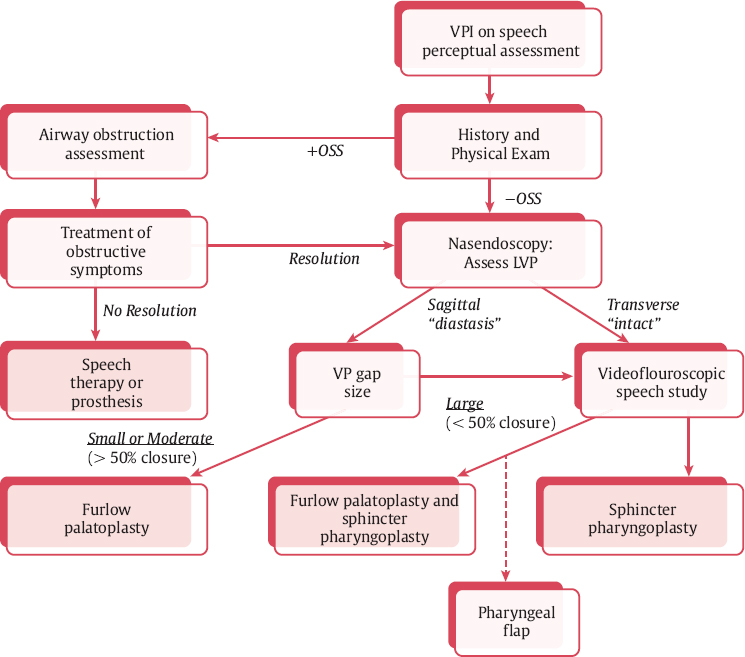
The authors use sphincter pharyngoplasty as the surgical procedure for treatment of VPD in patients with transverse orientation of the levator veli palatine. An algorithm for management as developed at Seattle Children′s Hospital is presented in Fig. 13.1 . In this chapter, we present the sphincter pharyngoplasty in detail. Sphincter pharyngoplasty is conceptually different from the posterior pharyngeal flap. It involves horizontal transposition of two vertical, lateral, superiorly based pharyngeal myomucosal flaps across the posterior aspect of the velopharynx to create a single central port.
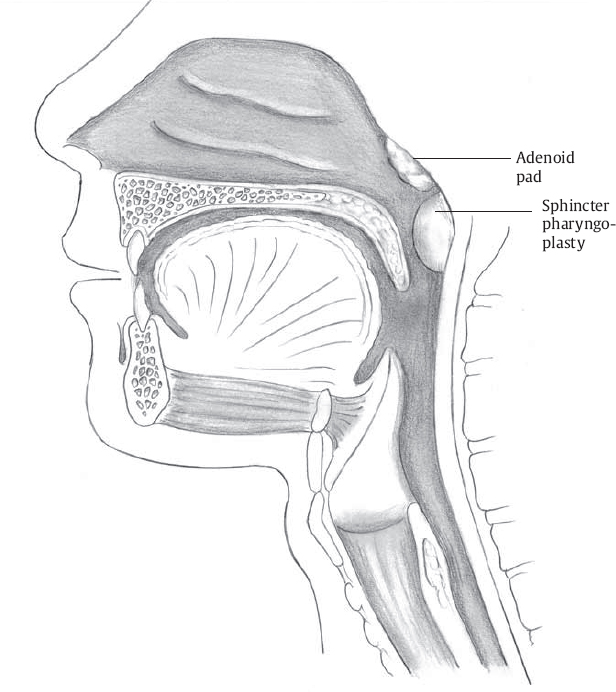
As suggested by its name, the goal of sphincter pharyngoplasty is to augment the velopharyngeal sphincter. Transposition of the pharyngeal myomucosal flaps essentially creates a “speed bump” at the caudal aspect of the velopharynx for the mobile velum to contact during speech ( Fig. 13.2 ). This procedure is particularly well suited for patients who demonstrate coronal velopharyngeal closure patterns ( Fig. 13.3 ). The benefit from sphincter pharyngoplasty is primarily attributed to augmentation of the posterior pharyngeal wall, as electromyographic studies evaluating dynamic muscular activity of the neo-sphincter have shown no intrinsic muscular activity.2
Historical Perspective
Sphincter pharyngoplasty has evolved since its original conception. In 1950, Hynes described elevation of bilateral superiorly based palatopharyngeus myomucosal flaps, rotated 90 degrees and sutured into a transverse mucosal incision made just inferior to the nasopharyngeal tori.3 Hynes performed this procedure in children older than 10 years in order to avoid the impediment of a hypertrophic adenoid pad. Additionally, he performed the procedure in children who had open soft palatal clefts; when the palate was intact, it was divided to improve nasopharyngeal exposure. Although several variations of this procedure have been proposed, the most notable was by Orticochea in 1968.4 This modification involved bilateral superiorly based flaps consisting of the posterior tonsillar pillars and underlying palatopharyngeus musculature being inserted into a small inferiorly based posterior pharyngeal flap within the oropharynx resulting in three ports. He further adapted the procedure to position the sphincter more superiorly.5 In 1985, Jackson adapted the Hynes procedure, transposing two superiorly based palatopharyngeal muscle flaps by 90 degrees at the level of the superior tonsillar poles.6 Finally, in 1998, Sie et al. presented a modification where the posterior palatopharyngeal flaps were elevated laterally to include the posterior tonsillar pillar, allowing for more superior positioning of the sphincter.7
In the last two decades, sphincter pharyngoplasty has become more commonly used to correct VPD, and in many studies is noted to have comparable or even favorable outcomes compared to the “workhorse” posterior pharyngeal flap procedure. The sphincter pharyngoplasty can be tailored by surgeons to suit the velopharyngeal deficit of the individual patient.
The Procedure
Preoperative Assessment and Planning
A thorough preoperative assessment of velopharyngeal function should precede decision for sphincter pharyngoplasty. As previously stated, Fig. 13.1 presents an algorithm for patient assessment and surgical management. This algorithm was developed at Seattle Children′s Hospital by the senior author (K.S.) and colleagues.
The preoperative assessment begins with a detailed patient history. The physician should specifically seek information pertaining to gross motor, speech, and neurologic development; SDB; otologic symptoms (suggestive of chronic eustachian tube dysfunction); nasal regurgitation of liquids; nasal air escape; and facial grimacing. A thorough patient and family history of cleft deformities should also be obtained, in addition to any history of syndromic abnormalities. In conjunction with the routine head and neck examination, the physician should assess for middle ear effusion or atelectasis, nasal obstruction, palatal abnormalities, tonsil size, tongue shape and mobility, and dysmorphic facial features.
A speech-language pathologist who is adept at evaluating velopharyngeal function in children should perform a complete speech assessment. Ultimately we consider a “speech differential diagnosis” assessing for all components of speech, essentially to identify speech behaviors such as childhood apraxia of speech, misarticulations, and velopharyngeal mislearning that may be unrelated to VPD.8 The child′s speech is evaluated with regard to voice, articulation, oral-motor sequencing, and velopharyngeal function. The speech differential further describes the main manifestations of velopharyngeal function: resonance, nasal air emissions, and velopharyngeal insufficiency (VPI)-related speech behaviors such as sound-specific VPI, nasal for nonnasal substitutions, compensatory misarticulations, and facial grimacing (see Chapter 11).
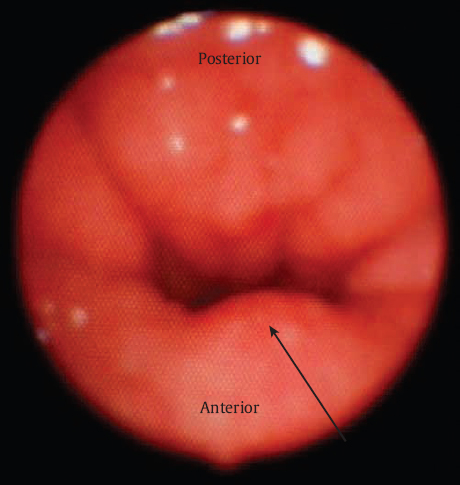
Following patient history, physical examination, and thorough speech evaluation, an instrumental assessment is performed. The instrumental assessment may consist of fiberoptic nasal endoscopy, multiview videofluoroscopy (MVF), or both. Strengths and limitations of these two procedures are discussed in detail in Chapter 11. The authors initiate instrumental assessment with nasal endoscopy via the middle meatus, where a bird′s-eye view of the velopharyngeal apparatus is obtained. The nasal surface of the soft palate is assessed for evidence of submucous cleft palate or sagittal orientation of the levator veli palatini musculature, most typically noted via a groove or midline diastasis in the palate ( Fig. 13.4 ). Notation is made of gap size in addition to degree of inward motion of the velum and lateral and posterior pharyngeal walls. A measurement scale for specific evaluation of velopharyngeal gap and motion such as the one suggested by Golding-Kushner and others may be applied, and has been shown to be reliable among raters.9,10 Adenoid or tonsillar obstruction is also noted during nasal endoscopy.
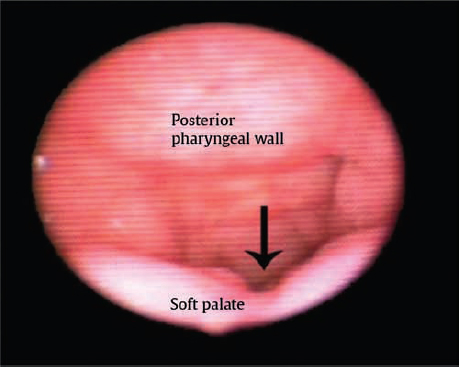
When there is no notch on the dorsal surface of the palate on endoscopic assessment, the patient will undergo evaluation with MVF, which is performed by the speech-language pathologist in the fluoroscopy suite. Barium drops are instilled into the nasal passage and fluoroscopic images of speech samples are obtained, typically in a total span of 80 to 120 seconds. MVF is particularly helpful in detailing the depth of the pharynx, the length of the velum, and the cephalocaudal point of contact between the velum and the posterior pharyngeal wall. MVF is also beneficial in clarifying specific patterns related to velopharyngeal mislearning (e.g., compensatory misarticulations) and paradoxical velopharyngeal movement. Limitations of MVF include the exposure to a small degree of radiation, and as it is an additional test to nasendoscopy it may be inconvenient for the patient. For these reasons MVF is not universally performed across institutions, but can provide information complementary to endoscopy.
The presence of a midline groove along the nasal surface of the soft palate can be indicative of sagittal-positioned levator veli palatini muscles and/or the presence of a submucosal cleft palate. If the instrumental assessment reveals no evidence of a midline groove, then sphincter pharyngoplasty is performed to correct VPD. Furlow palatoplasty is performed as the primary procedure of choice when a groove is identified and the velopharyngeal gap is < 50%. If the gap is > 50% in conjunction with a groove or suspected submucous cleft palate, palatoplasty is performed concomitantly with sphincter pharyngoplasty.
Sleep-disordered Breathing and Tonsillectomy
The patient history and physical examination should also identify the presence of upper airway obstruction or sleep-disordered breathing (SDB). Symptoms of snoring, rhinosinusitis, open-mouth breathing, or prior diagnosis of obstructive sleep apnea (OSA) should be ascertained. On examination, the oropharynx should be assessed for presence of tonsillar hypertrophy. For any child where SDB is suspected based on history and physical, preoperative polysomnography is recommended to evaluate for OSA. This evaluation is particularly important given the potential risk of postoperative SDB/OSA from any VPD surgical procedure. Additionally, significant tonsillar hypertrophy may exacerbate VPI by impeding velopharyngeal closure due to anatomic obstruction of the mobile velum. Children with obstructive symptoms and tonsillar hypertrophy should undergo tonsillectomy as a staged procedure 2 to 3 months prior to planned sphincter pharyngoplasty. We generally plan for repeat nasoendoscopy 6 to 8 weeks after tonsillectomy to reassess the pattern and size of the velopharyngeal gap prior to sphincter pharyngoplasty.
Large tonsils and adenoids may also impair exposure of the posterior pharyngeal wall and nasopharynx intraoperatively. In the event where adenotonsillar hypertrophy is noted but the child has no SDB symptoms or polysomnographic evidence of OSA, adenotonsillectomy can be performed at the same time of sphincter pharyngoplasty in order to facilitate surgical exposure. The authors prefer this approach only when SDB is not a preoperative concern.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree