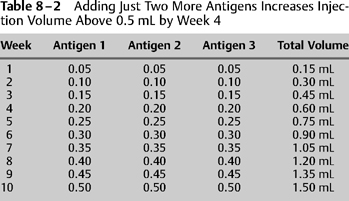
8 The objective of immunotherapy is to alter the level of tolerance to allergenic stimulation. This is achieved through the systemic administration of antigens that have been proven to cause immunoglobulin E (IgE)-mediated hypersensitivity. Two polarized goals in this effort are to achieve symptom relief quickly and at the same time avoid anaphylaxis. Paramount to these goals is the proper administration and interpretation of quantitative testing and the proper preparation of treatment vials based on these results. Regardless of which test methods are chosen to prove IgE-mediated allergy, in the end the clinician must find a dosing schedule that is safe and efficient. In the ideal world the actual dose at which to begin immunotherapy depends on the results of precise intradermal dilutional testing (IDT) as described previously in this text. IDT defines the endpoint for each positive antigen tested. The endpoint represents the antigen dose at which the patient first exhibits local mast cell degranulation. The endpoint represents the amount of antigen that begins to stimulate the immune system through local anaphylaxis without causing systemic anaphylaxis. An immunotherapy dosing schedule that begins in this endpoint dilution range is safe and will theoretically lead to quicker immunotolerance and symptom relief than will be achieved with weaker starting dose schedules. In the real world, less precise forms of quantitative tests are often used to predict the IDT endpoint; however, in theory, the clinician should always use these other test results to ascertain what the endpoint would have been had IDT been used. The clinician should ask the question: “If I had performed IDT instead of radioallergosorbent testing (RAST) or modified quantitative testing (MQT), what would have been the endpoint?” Experience and history have shown that a subcutaneously administered starting dose of 0.05 mL of the endpoint dilution is safe and builds the path for immunotherapy efficiency. However, it should also be safe in theory because historically these patients were nearly all IDT-tested, and during this testing, antigen amounts totaling more than 0.05 mL of the endpoint dilution antigen were already administered. This is because 0.01 mL of the dilution that was one dilution stronger than the endpoint dilution was safely administered as a confirmatory wheal during IDT. The antigen amount in this confirmatory wheal was equivalent to 0.05 mL of the endpoint dilution. Adding to that the 0.01 mL of endpoint dilution already given leads to the conclusion that more than 0.05 mL of endpoint antigen units have already been given to the patient during testing.1,2 In the early days, there were not many antigen extracts available, and thus the clinician had a comparatively easy task of injecting starting doses of 0.05 mL of one or two antigens by drawing up in a syringe their corresponding endpoint dilutions from the IDT board (Table 8-1). However, as time went on and numbers of available antigens increased, and the practice of drawing up of solutions of antigens from the test board for each patient each week waned (Table 8-2), because therapy with multiple antigens required either several injections of small volumes or single injections of large volumes, neither of which was acceptable to patients. How can this problem be solved? The discussion that follows describes a simple and well-accepted method of administering injections from a treatment vial prepared specifically for each patient. Such a vial (1) requires beforehand preparation only once for many injections; (2) contains multiple antigens; (3) delivers these multiple antigens in a single injection; (4) maintains the volume of injections in a range of 0.05 to 0.50 mL; (5) allows each antigen strength to be individualized according to its assigned endpoint; and (6) allows for the equivalent of 0.05 mL of the endpoint dilution of each individual antigen to be administered as the starting dose.
Vial Preparation Based on Quantitative Testing
Richard C. Haydon III
♦ IDT-Based Subcutaneous Immunotherapy Starting Doses
♦ IDT-Based Preparation of Multidose, Multi-Antigen Treatment Vials
|
Testing and Treatment Boards
For smaller allergy practices, and for those just starting, time and expense can be saved by using the same set of antigens for intradermal testing and for preparation of treatment vials. But because the intradermal testing antigens are diluted with phenolated normal saline (PNS) or human serum albumin (HAS), dilutions Nos. 2 to 6 in the test board contain less than a 10% concentration of glycerine. Thus, the potency may diminish after 6 to 8 weeks (and even sooner if not properly refrigerated!). Therefore, when one board is used for both intradermal testing and treatment vial preparation, it is important that the board be kept refrigerated as much as possible, and that it be replaced with newly prepared dilutions every 6 to 8 weeks.2–4
The creation of a completely separate treatment board for the sole purpose of preparing treatment vials is an option for the very busy allergy practice. This may be especially advantageous when mixing treatment vials becomes a nearly full-time job. A separate mixing board minimizes cross-traffic, needle entries, and premature depletion of certain dilutions. And because the treatment board is not used for testing, one can prolong the potency of even the weakest dilutions to 12 months or more by adding enough glycerine to create a 25 or 50% concentration.1,4 (Glycerine should not be added to dilutions to be used in intradermal testing, due to the irritative, painful, and whealing enhancement properties known to be associated with glycerine.)
It is important to note that the vial mixing examples that follow assume that the one-board concept, using the same antigens in both intradermal testing and treatment vial preparation, is being utilized. Therefore, these examples assume that no extra glycerine has been added to the dilutions created for use on this combination test and treatment board.
Directions for IDT-Based Preparation of Immunotherapy Treatment Vial (Table 8-3)
- Select the antigens to be included for immunotherapy, based upon history, physical examination, and IDT.
- From the test board, in separate syringes, draw up 0.2 mL of each antigen, from the dilution that is positioned two dilutions to the right (two dilutions stronger) of the endpoint dilution for each antigen selected.
- Put these antigens in a sterile 5-mL glass treatment vial.
- Add enough diluent (usually PNS) and 50% glycerine (usually 1.0 mL for potency preservation; see below) to increase the total volume of antigens +diluent +glycrine in the treatment vial to 5.0 mL.
- Put these antigens in a sterile 5-mL glass treatment vial.
| Antigen | Endpoint | Multidose Vial |
| Ragweed | 5 | 0.2 mL No. 3 |
| Pigweed | 4 | 0.2 mL No. 2 |
| Timothy | 6 | 0.2 mL No. 4 |
| Maple | 6 | 0.2 mL No. 4 |
| Total antigen Volume | 0.8 mL | |
| Add 50% | Glycerine 1.0 mL | |
| Add Diluent | 3.2 mL | |
| Total: | 5.0 mL |
After performing the safety vial test and obtaining the proper results, one can then administer a 0.05-mL injection from this treatment vial, which is equivalent to 0.05 mL of the endpoint dilution for each individual antigen.
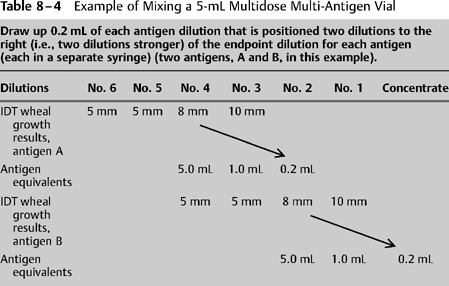
For those who wish to prove the concept just described, consider the following (Tables 8-4 and 8-5):
- During IDT a patient’s endpoints are on a No. 4 dilution.
- You could give the patient a starting dose consisting of 0.05 mL of the No. 4 dilution.
- But … to accomplish the goal of small-volume injections containing multiple antigens, a better alternative follows:
- Multiply the starting dose amount (0.05 mL of the No. 4) by 100, now giving a volume of 5.0 mL of No. 4. This amount now contains 100 starting doses.
- Due to the fivefold dilution concept used in preparing test dilutions, recall that the number of antigen particles in 5.0 mL of a No. 4 dilution is the same as is contained in 1.0 mL of a No. 3 dilution or 0.2 mL of a No. 2 dilution (one-fifth the volume in each case).
- In other words the amount of antigen in 5.0 mL of a No. 4 dilution is the same as that contained in a volume 1/25th as great of a dilution that is 25 times stronger (0.2 mL of the No. 2 dilution).
- See item 4 above. If the 0.2 mL of a No. 2 dilution were injected into the patient, it would result in a dose that was 100× the starting dose.
- Therefore, if we choose to use the No. 2 dilution to start immunotherapy, we must figure out a way to decrease the antigen amount to be given to the patient by a factor of 100.
- To solve this problem, begin by putting the 0.2 mL in a 5-mL sterile glass treatment vial. Then decrease the antigen concentration by a factor of 25 by adding 4.8 mL of diluent. (This is one part antigen and 24 parts diluent, which is 1: 25.) Now the total volume in the treatment vial is 5.0 mL.
- If 0.2 mL from this treatment vial were now injected into the patient, it would result in a dose that is 4× the starting dose.
- Therefore, we must figure out away to decrease the antigen amount to be given to the patient by a factor of 4.
- This is accomplished by, instead of injecting 0.2 mL, administering 0.05 mL from the treatment vial. This decreases the antigen amount by a factor of 4.
- Now, 0.05 mL of volume drawn from the treatment vial mixed accordingly will deliver a dose that is antigenically equivalent to 0.05 mL of the endpoint dilution.
- See item 4 above. If the 0.2 mL of a No. 2 dilution were injected into the patient, it would result in a dose that was 100× the starting dose.