© Springer International Publishing Switzerland 2015
Fiona Zwald and Marc D. Brown (eds.)Advances in Transplant Dermatology10.1007/978-3-319-12445-2_11. Update on Benign and Inflammatory Skin Disease Secondary to Transplant Medication
(1)
Department of Dermatology, Duke University Medical Center, Durham, NC, USA
1.1 Introduction
1.5 Viral Infections
1.8.1 Glucocorticoids
1.8.2 Cyclosporine
1.8.3 Tacrolimus
1.8.4 mTOR Inhibitors
1.8.5 Azathioprine
1.8.6 Mycophenolate
1.9.1 Retinoids
1.9.2 Voriconazole
1.9.3 EGFRI
1.10 Summary
Abbreviations
APC
Antigen-presenting cell
AZA
Azathioprine
EGF
Epidermal growth factor
EGFRI
Epidermal growth factor receptor inhibitors
FKBP12
FK506-binding protein
GVH
Immune-mediated graft failure
HHV
Herpes virus
HPV
Human papillomavirus
HSV
Herpes simplexvirus
HUS
Hemolytic uremic syndrome
MAPK
The RAS-mitogen-activated protein kinase pathway
MC
Molluscum contagiosum
MM
Mycophenolate mofetil
mTOR
Mammalian target of rapamycin
NMSC
Nonmelanoma skin cancer
SD
Seborrheic dermatitis
SH
Sebaceous hyperplasia
SOTR
Solid organ transplant recipients
1.1 Introduction
Solid organ transplantation is the treatment of choice for many patients with organ failure. At the end of 2010, over 260,000 organ transplant recipients were alive in the United States. More than 25,000 new organs were transplanted in 2011, the majority of which were kidney (16,055), liver (5,805), lung (1,830), and heart (1,949) [1]. Over the past 20 years, the number of solid organ transplantations performed annually and the 5-year graft survival have increased, both in large part due to safer and more effective immunosuppressive medications. The demographics of organ transplant recipients have also evolved with more transplantations being performed in patients aged 65 years or older and in minority groups [1]. These data indicate that not only are more people living with an organ transplant but the transplanted population is increasing in diversity. Furthermore, skin disorders occur at a greater frequency in solid organ transplant recipients (SOTR) largely due to immunosuppressant medications, which are generally successful in their therapeutic effect of suppressing the immune system. However, immunodeficiency, along with direct toxic effects of many medications, often causes cutaneous disorders. A dermatologist should be aware of the changing demographics and unique therapeutic factors affecting this patient population.
1.2 Immunosuppressives: Mechanism of Action
T cells are the main drivers of immune-mediated graft failure (GVH) and are, the major target of immunosuppressant medications [2]. Clinical immunosuppression typically occurs in three phases: induction therapy, maintenance therapy, and therapy of an acute rejection.
Induction therapy is targeted at depleting T cells to prevent early, acute rejection. This therapy usually takes place in close proximity to transplantation, starting either during the intraoperative or immediately postoperative period, and is completed within the first 1–2 weeks after transplantation. The most commonly used induction agents are either antibodies that deplete T cells or IL-2 receptor antagonists that prevent T cell activation [3–5]. Induction therapy is not always used; in 2011, close to half of lung, liver, and heart transplant recipients did not receive induction therapy. However, over 80 % of kidney transplant recipients did receive induction, most commonly a T cell–depleting agent [1]. Induction therapy may cause prolonged T cell depletion, with increased risk for opportunistic infections, post-transplantation lymphoproliferative disorder, and possibly autoimmune disease.
Maintenance immunosuppressive agents are often life-long therapies [6]. Maintenance therapies generally work by interfering with one of the three signals required for T cell activation and proliferation. Signal 1 occurs through the interaction between an antigen-presenting cell (APC) and the CD-3 receptor on a T cell. Signal 2 occurs when a costimulatory complex is formed between CD80 and CD86 on the APC and CD28 on the T cell. Together, these two signals activate three signal transduction pathways: the calcium-calcineurin pathway, the RAS-mitogen-activated protein (MAP) kinase pathway, and the nuclear factor-κB pathway [7]. Once activated, these pathways stimulate the expression of numerous inflammatory mediators, including cytokines, in particular interleukin-2. Il-2 and other cytokines activate the “target of rapamycin” (mTOR) pathway that triggers cellular proliferation, which constitutes signal 3. B-cells can also be involved in alloimmune responses, if their receptors are engaged by antigen, through the production of alloantibody against donor HLA antigen [8, 9]. Five main classes of the most commonly used maintenance therapies are calcineurin inhibitors, co-stimulation blockers, mammalian target of rapamycin (mTOR) inhibitors, antiproliferatives, and corticosteroids [10]. Given the diverse pathways involved in alloimmunity, combination immunosuppressive therapy is usually required for successful suppression of alloimmune responses.
Since 1980, several significant advances toward more effective and less toxic maintenance immunosuppression have made transplantation more successful. In early transplant medicine, azathioprine (AZA), which inhibits purine synthesis, was used for maintenance immunosuppressive medication until cyclosporine largely replaced it in the early 1980s [11]. Cyclosporine was much more effective at reducing rejection rates and improving 1-year graft survival, which made transplantation more successful. Cyclosporine works by forming a complex with cyclophilin, inhibiting the calcineurin pathway and decreasing the synthesis of key cytokine genes that promote T cell activation and proliferation [6, 12]. Cyclosporine has many adverse effects including nephrotoxicity, hemolytic uremic syndrome (HUS), hypertension, hyperlipidemia, diabetes mellitus, gingival hyperplasia, hirsutism, and skin changes [13]. In the 1990s, tacrolimus, another calcineurin inhibitor, was introduced. Tacrolimus blocks the calcineurin pathway by binding FK506-binding protein (FKBP12), a more potent inhibitor of calcineurin than cyclosporine. Tacrolimus is more likely to cause DM than cyclosporine and can similarly cause nephrotoxicity and HUS but is less likely to cause hyperlipidemia, hypertension, and skin changes [14]. Today, tacrolimus is the most frequently used CNI in maintenance immunosuppression due to its superiority in preventing early acute rejections; however, patient survival, graft function, infection, malignancy, and blood pressure profiles are similar to cyclosporine [1, 15, 16].
The next significant advancement in maintenance immunosuppression occurred in the early 1990s with the introduction of mycophenolate mofetil (MM). MM inhibits purine synthesis by blocking inosine monophosphate dehydrogenase, leading to a decrease in lymphocyte proliferation. Although it acts similarly to AZA, its use in combination with CNIs led to improved patient and graft survival and decreased early and late allograft rejection [17, 18]. Furthermore, MM has a much better side-effect profile and requires less monitoring, which is why it has largely replaced AZA and is used in the majority of maintenance regimens today.
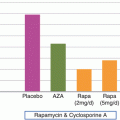
More recent advances in immunosuppression came with the introduction of mTOR inhibitors, sirolimus, and everolimus. These drugs are macrolide antibiotics that bind FKBP12 and inhibit cytokine (IL-2) activation of the mTOR pathway, blocking lymphocyte activation and proliferation. Although they bind FKBP12 as tacrolimus does, they function independently of the calcineurin pathway [19, 20]. Their principle adverse effects include hyperlipidemia, thrombocytopenia, and impaired wound healing, but they can also cause mouth ulcers and skin lesions [21]. Although they were initially developed to use as combination therapy with cyclosporine, they can also potentiate cyclosporine’s adverse effects including nephrotoxicity and incidence of HUS and hypertension [22, 23]. Combination immunosuppression with tacrolimus and sirolimus has also resulted in more renal dysfunction and hypertension than tacrolimus plus MM [24, 25]. These features, along with minimal data to support net benefit over harm, have deterred the widespread use of mTOR inhibitors in maintenance immunosuppression [15]. Of note, mTOR inhibitors have anti-angiogenesis effects, which may benefit SOTR who develop de novo cancer (including skin cancer) or those who have a high risk of developing cancer [15, 26].
Belatacept, which blocks the co-stimulation of T cell (CD28) by APCs (CD80/86), has been recently approved for maintenance immunosuppression in renal transplant patients. Randomized, controlled trials comparing the efficacy of belatacept to cyclosporine demonstrated similar patient and graft survival, with better renal function compared with cyclosporine [27–30]. The unintended consequences of co-stimulation blockade have not been fully elucidated; therefore, whether belatacept replaces CNIs in maintenance immunosuppressive regimens remains to be seen [31].
In summary, the most frequently used combination immunosuppressive therapy is currently tacrolimus + MM +/− corticosteroids [15]. Lung and heart SOTR require the most immunosuppression, while liver transplant recipients often require less immunosuppression overall and tolerate early minimization therapy [1].
1.3 Skin Disease in SOTR
Aside from the kidney disease, hypertension, hyperlipidemia, diabetes mellitus, and HUS caused by immunosuppressant medications, skin disease, both benign and malignant, affects a large portion of STOR. Much research and focus has been placed on the marked increase in development of cutaneous malignancies; however, benign and inflammatory skin diseases are common in this population and can have a tremendous impact on quality of life and medication compliance. A broad spectrum of skin conditions affects SOTR, and many studies have estimated the prevalence of cutaneous manifestations in SOTR to range from 12.5 to 71 % [32–36]. Several factors affect the incidence of skin disease in SOTR including patient characteristics (age, gender), phototype, sun exposure, previous viral exposures, HLA typing [37–41], type of transplantation, level of immunosuppression, and specific medication regime. The type of skin disorders also changes with time, with cutaneous infections and inflammatory disorders predominating during the early years and premalignant and malignant lesions increasing in incidence concordantly with time from transplant [36, 37, 42, 43].
1.4 Cutaneous Infections
Overall, the most frequently encountered cutaneous infections in transplant recipients are superficial fungal infections and viral warts. However, modification of immunosuppression over time influences both the susceptibility to and prevalence of infections in SOTR. Overall immunosuppression is determined by the number, dose, duration, and sequence of immunosuppressive medications [42]. Within the first month after transplantation, the most frequently encountered cutaneous infections are nosocomial, including MRSA and wound- and catheter-associated infections, and reactivation of previously acquired infections. Less common infections include HSV and overgrowth of colonizers Aspergillus and pseudomonas [42]. Opportunistic infections are uncommon in the first postoperative month, which highlights the role that duration of immunosuppression has in the susceptibility to infection [44]. The intermediate period from 1 to 6 months post-transplant is the most risky for the development of opportunistic infections as patients are typically on high immunosuppressant doses.
1.5 Viral Infections
Given the suppression of T cell immunity and the role of T lymphocytes in combating viral pathogen [45], viral infections tend to predominate 1–6 months post-transplantation [42]. Antiviral prophylaxis has significantly decreased the incidence of herpes virus (HHV) infections that historically occurred between 1–6 months post-transplantation [46, 47]. However, reactivation of HHV represents a significant risk in this patient population and is important to diagnose and treat. The most common HHV infections in SOTR are herpes simplexviruses (HSV) types 1 and 2, cytomegalovirus, Epstein-Barr virus, HHV-6, HHV-7, and HHV-8, which is the causative agent of Kaposi Sarcoma [43, 48]. HSV infection is most commonly manifested through mucocutaneous lesions in the organ transplant population and must be distinguished between sirolimus-induced mucositis in the appropriate setting [43, 49]. The risk of HSV infection decreases 6 months after transplantation as immunosuppression is tapered. However, when lesions develop, they can be painful, persistent, and rarely disseminate to distant organs [50]. The most common treatments are acyclovir and valacyclovir, which can be used acutely and as suppressive therapy. Alternatives for acyclovir-resistant HSV include foscarnet, cidofovir, and trifluridine [51–54]. In patients with prolonged or high-dose immunosuppression, such as lung-transplantation recipients or those with frequent episodes of rejection, consideration should be given to chronic suppressive antiviral therapy. Moreover, given the increased incidence and impact on quality of life of viral infections, routine antiviral prophylaxis may be reasonable for the majority of SOTR.
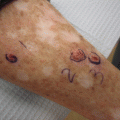
After the sixth postoperative month, SOTR continue to be at high risk for developing community-acquired infections, and over time more indolent infections begin to develop, which are frequently caused by human papillomaviruses (HPV) [55–59]. In a recent prevalence study of renal transplant recipients, the most common cutaneous infection was viral warts, which was found in 38 % of patients and increased in frequency in concordance with duration from transplant. These results were consistent with several previous studies [8, 60–63]. HPV in SOTR can be challenging to treat and is an additional risk factor for NMSC, especially in this patient population [64]. Treatment can either be attempted through physical destruction (cryotherapy, curettage, laser) or topical therapies including salicylic acid, podophyllum, cantharidin, trichloroacetic acid, topical cidofovir, topical vitamin D, retinoids, or imiquimod [43, 65]. Imiquimod is not approved for use in transplant patients but has been shown to be very effective in enhancing cytokine production and indirectly stimulating T cell activity, thereby increasing patients’ ability to effectively eliminate viral lesions [66–68]. Progressive or symptomatic disease may warrant systemic therapy such as intravenous cidofovir [69, 70]. Even with clearance, viral warts are a chronic condition and recurrence should be anticipated.
Less frequently, molluscum contagiosum (MC), a poxvirus that infects squamous epithelia [71, 72], has been shown to occur in nearly 7 % of pediatric SOTR [73], extensively in immunocompromised patients due to HIV, corticosteroid use, or chemotherapy [74]. Atypical presentations in SOTR have also been reported including multiple, giant MC lesions and MC folliculitis [75–77]. MC can be treated by surgically removing the umbilicated core, cryoablation, electrodessication, cantharidin, trichloroacetic acid, podophyllum, and topical tretinoin [43, 78]. For more extensive cases, topical imiquimod or cidofovir gel may be used [43, 79, 80]. Other rare viral infections include viral-associated trichodysplasia spinulosa and acquired epidermodysplasia verruciformis [81, 82].
1.6 Fungal Infections
Cutaneous fungal infections also occur frequently due to the suppression of cell-mediated immunity and, possibly, a reduction in the concentration of APC in the epidermis and can vary greatly in presentation [83]. In the early post-transplantation period, when immunosuppression is greatest, disseminated candidiasis and aspergillosis, both primary and secondary cutaneous resulting from invasive disease, are frequently seen but with institutional variability in incidence. Immunosuppressive doses also tend to be high 1–6 months post-transplantation, and patients continue to be at significant risk of developing an opportunistic deep fungal infection. Common mycoses include dimorphic fungi infections, zygomycoses, phaeohyphomycosis, hyalohyphomycosis, and cryptococcosis as well as continued risk from Candida and Aspergillus. Skin lesions range from nonerythematous papules, erythematous macules, papules and nodules (+/− necrosis), hemorrhagic bullae, abscesses, and cellulitis. Cutaneous fungal infections may occur in isolation or be a clue to underlying systemic infection. Early recognition of systemic infection is imperative given the potential mortality associated with disseminated disease [84, 85]. As clinical presentation is variable and relatively nonspecific, a high index of suspicion and early biopsies of skin lesions for histologic examination and culture are critical for diagnosis. Therapy usually requires systemic antifungal compounds including triazoles such as voriconazole and posaconazole, amphotericin B, and echinocandins such as caspofungin, micafungin, and anidulafungin, but surgical debridement/excision may be an adjunct in localized disease.
Overall, superficial fungal infections represent more common infections in SOTR. Mucocutaneous candidiasis is frequent, especially early in post-transplantation [86]. Nystatin or topical azoles are commonly used as effective treatments for superficial Candida infections. Dermatophyte infections often present atypically due to a lack of erythema that is normally produced by local inflammation [87]. Pityriasis versicolor has been identified as the most common fungal infection in SOTR in several studies [88–92] and can be treated with topical or oral medications, most commonly topical azole antifungals. In more involved cases, oral itraconazole can be very effective, but drug interactions with immunosuppressant medications must be considered and discussed with the transplant team [93]. Other frequent infections include chronic tinea pedis and onychomycosis, both of which are more common with chronic immunosuppression [59, 88–90, 94]. Oral terbinafine is the treatment of choice and has the benefit of little interaction with immunosuppressants, but liver function should be monitored during therapy [95]. Majocchi’s granuloma is a deep dermal folliculitis caused by dermatophytes that tends to occur more frequently on the lower extremity in males who are on chronic (>36 months) immunosuppression [96, 97]. The causative agent of Majocchi’s granuloma is most frequently Trichophyton rubrum, but other fungal species have also been isolated. Regardless of the causative agent, systemic therapy is often utilized, many times in conjunction with topical antifungals [96].
1.7 Common Inflammatory Disorders and Benign Tumors
Although several of the inflammatory dermatoses encountered in SOTR are due to drug effects, several conditions arise from an immunosuppressed state or hypersensitivity reaction, including folliculitis, drug rash, keratosis pilaris, hair loss of telogen effluvium type, sebaceous hyperplasia, seborrheic dermatitis (SD), acrochordons, porokeratosis, and seborrheic warts [59]. Several of these disorders, including keratosis pilaris and SH, have a similar prevalence in the non-transplant population and, therefore, may be unrelated to transplantation in some SOTR [98–100]. Skin tags have been shown to increase with age, duration from transplant, and BMI of SOTR, which may also be unrelated to transplantation [59]. Folliculitis has been reported in many SOTR, more frequently in early post-transplantation period, in males, and with cyclosporine use [35, 36, 59]. SD has been reported to occur in 9.5 % of renal transplant recipients [101], which is much higher than SD prevalence of 1–5 % reported in the normal adult population [102, 103]. However, SD occurs at a higher rate in other immunosuppressed populations such has HIV patients [104–106], patients with carcinomas of the upper respiratory and GI tracts associated with heavy alcohol and tobacco use [107], patients undergoing PUVA [108], and mountain guides with excessive sun exposure [109]. Although the precise etiology of SD is unclear, the commonality of immunosuppression in each of these populations suggests impaired T cell immunity leading to an altered immune response to Malassezia species and more prevalent SD [101]. Treatment with combination topical antifungals, steroids, or calcineurin inhibitors remains the most effect therapy for SD in SOTR [101].
Porokeratoses are thought to arise from abnormal clonal expansion of keratinocytes and, in SOTR, typically present as an isolated, annular plaque with central atrophy and a hyperkeratotic rim but can also present in superficial disseminated forms [110–113]. The incidence of porokeratoses in SOTR, from two population-based studies, is between 8 and 10.68 % [112, 114]. Immunosuppression has been suggested to play a role through impairment of immune surveillance by Langerhans cells [115], and the development of porokeratoses has been directly linked to the strength of immunosuppressive therapy [116–118]. The risk of malignant transformation of porokeratosis to SCC in the general population is approximately 7 %, which may be greater in SOTR secondary to immunosuppression, larger lesion sizes, and configuration [119]. Treatment of porokeratosis can present a challenge and is usually approached through topical chemotherapies including 5-fluorouracil or imiquimod, photodynamic therapy, and chemical peels; disseminated disease requires systemic therapy with oral retinoids [111].
Seborrheic warts are common in SOTR and, although their relationship with HPV status is unclear, a significant relationship between the quantity of seborrheic warts and NMSC, particularly BCC, has been established in SOTR [120]. Seborrheic warts are also more prevalent in lighter-skinned, older patients who had been transplanted the longest [120]. In patients with numerous seborrheic warts, acitretin can be helpful in decreasing lesion load [121].
1.8 Specific Drug-Induced Skin Changes: Immunosuppressants
1.8.1 Glucocorticoids
Aside from predisposing SOTR to infectious and inflammatory skin conditions, most immunosuppressive medications have direct toxic effects on the skin that vary with dose and duration of therapy. Historically, glucocorticoids were used at much higher doses compared with newer regimens; in fact, while many patients remain on chronic low doses, some immunosuppressive regimens have transitioned to steroid-free protocols [1]. The most frequent cutaneous complications of systemic steroids are acne, Cushingoid features, purpura, skin atrophy, and striae. Steroid-induced acne is dose-dependent and tends to present with monomorphic inflammatory papules and pustules on the trunk and upper extremities. This eruption is a neutrophilic folliculitis rather than true acne [122]. Treatment consists of decreasing the glucocorticoid dose and a trial of topical therapies including benzoyl peroxide, erythromycin, and clindamycin [123]. Oral therapies, including doxycycline and other oral antibiotics similarly used for acne, spironolactone in non-child-bearing women and isotretinoin, may be required for more resistant cases [85]. Glucocorticoids lead to redistribution of fat to the face, neck, and trunk characteristic of Cushingoid appearance, which is frequent in the immediate post-transplantation period when higher doses of systemic steroids are often used; as these doses are tapered, the Cushingoid features may or may not resolve. Purpura, related to skin atrophy, is also frequently seen in patients on long-term glucocorticoids and may be minimized using topical retinoids (0.01–0.05 %) or ammonium lactate (6–12 %) [85]. Striae, mainly located over the abdomen, thighs, and buttocks, may develop in patients on high-dose or long-term glucocorticoid therapy, particularly children and women. Striae are difficult to treat and no high-quality studies have found effective treatments [73, 124], but topical retinoids or pulsed dye laser may be helpful in early erythematous lesions [125, 126].
1.8.2 Cyclosporine
Cyclosporine has largely been replaced by more effective and safer immunosuppressant medications. Furthermore, the doses are lower than those historically used. These trends have decreased the frequency of cutaneous adverse effects, which tend to be dose-dependent. The most common cutaneous effects of cyclosporine include frequent hypertrichosis, possibly through increased activity of alpha reductase, leading to increased levels of dihydrotestosterone in peripheral tissues [127, 128]. Pilosebaceous lesions are also a common development as cyclosporine may be partially eliminated through sebaceous glands and pathogenic in folliculodystrophy [129]. Clinical lesions include sebaceous hyperplasia (SH), predominantly in male transplant patients; epidermal cysts; keratosis pilaris; and more nonspecific follicular eruptions [130–133]. Most of these conditions do not require intervention, but for extensive or irritated SH, intralesional desiccation [134], photodynamic therapy [135, 136], bichloracetic acid [137], and isotretinoin [138, 139] have been helpful in lesion reduction. Rarely, recalcitrant hyperplastic folliculitis and pilomatrix dysplasia have been reported [140–142]. Gingival hyperplasia is another well-known side effect, which occurs in approximately 1/3 of patients and generally begins after 3 months of therapy. It can be exacerbated by poor oral hygiene or concomitant use of phenytoin and calcium channel blockers [59, 85, 143]. Azithromycin may improve overgrowth and clinical appearance [144, 145].
1.8.3 Tacrolimus
Despite numerous potential systemic side effects, very few reports of cutaneous side effects related to tacrolimus have been published. Several cases of alopecia, more commonly occurring in females, have been reported and respond to either decreased dosage or alternative medication [146–148]. Interestingly, although topical tacrolimus is used in general dermatology for atopic dermatitis, tacrolimus-induced atopic dermatitis has been reported in rare cases when tacrolimus is used as a systemic immunosuppressant [149, 150]. Multiple epidermal cysts erupting in a patient on systemic tacrolimus has also been reported [151].
1.8.4 mTOR Inhibitors
Sirolimus and everolimus are immunosuppressive alternatives, especially in SOTR who develop numerous cutaneous squamous cell carcinomas. Sirolimus is more commonly used and associated with specific cutaneous side effects. The most frequent side effect is aphthous ulcerations, which can be severe [152–155]. Mucosal ulcerations occur more frequently with loading doses of sirolimus, which suggests a correlation between ulcerations and sirolimus dose. The ulcerations are largely self-limiting but can be a recurring problem [152]. When aphthae occur, ensuring the level of sirolimus is within the therapeutic range can be helpful. Aggressive use of potent topical steroids may also aid in resolution [152]. Pilosebaceous dermatoses also occur, most frequently presenting as an acne-like eruption within 1 month of starting sirolimus [49, 156–158]. These eruptions occur in nearly 50 % of patients, with a strong male predilection, and consist mostly of inflammatory papules, pustules, and occasional painful nodules involving the face and trunk [156, 157]. A possible mechanism for the pathogenesis of this inflammatory eruption is inhibition of the epidermal growth factor (EGF) pathway [159]. Topical therapy may be helpful, but systemic therapy with oral antibiotics is often required, and discontinuation of sirolimus may be necessary for recalcitrant cases [156, 158]. Leukocytoclastic vasculitis due to sirolimus therapy is rarer but also requires discontinuation of sirolimus [155, 160, 161]. Disorders of the nail, consisting of onycholysis, erythema, splinter hemorrhages, and pyogenic granulomas have also been reported with sirolimus [153]. Similar effects have been seen in patients transitioned to everolimus due to increased incidence of NMSC [162, 163].
1.8.5 Azathioprine
Minimal cutaneous adverse effects may be seen with AZA, mostly affecting the hair, including thinning of the hair or changes in hair color and texture [164, 165]. An erythema nodosum hypersensitivity reaction has also been reported in patients receiving AZA for inflammatory bowel disease, which may have been a result of the underlying illness rather than a direct effect of AZA [166].
1.9 Specific Drug-Induced Skin Changes: Other
Several non-immunosuppressant medications with cutaneous side effects may also be encountered during long-term care of transplant patients including retinoids, voriconazole, and epidermal growth factor receptor inhibitors (EGFRI).
1.9.1 Retinoids
Dermatologists caring for SOTR frequently encounter early onset and high tumor burden of NMSC and often utilize oral retinoids as a management strategy. Retinoids promote cellular differentiation, help control epithelial growth, downregulate proto-oncogenes, and can be a very effective chemoprevention for NMSC [170–172]. Adverse cutaneous effects of oral retinoid therapy are well documented, largely dose-dependent, and include mucocutaneous xerosis, the most common manifestations being cheilitis, generalized xerosis, palmoplantar desquamation, hair loss, nail dystrophy, eczema exacerbation, and skin fragility [170, 173, 174]. Initiating acitretin at 10 mg per day and increasing by 10 mg increments at 2–4 week intervals to desired effect helps minimize side effects and improve patient compliance [175].
1.9.2 Voriconazole
Voriconazole is frequently used as a prophylactic antifungal therapy and for the treatment of severe fungal infections, especially in lung transplant recipients. Voriconazole can have adverse cutaneous effects in up to 8 % of patients, with phototoxicity being reported most frequently, but cheilitis (often associated with phototoxicity), worsening of psoriasis, alopecia, and pruritus are also reported [176]. The clinical presentation and time course of voriconazole-induced phototoxicity are variable. Clinical lesions have been described as erythema, erythema plus cheilitis, erythema plus lentigines, erythema plus keratosis, bullous erythema, and pseudoporphyria. Regardless of the clinical presentation, the lesions are photodistributed, involving face, upper chest, arms, hands, and legs [176]. The photodistributed erythema and associated pigmentation changes suggest a phototoxic mechanism for the photosensitivity [177, 178]. A genetic polymorphism that decreases metabolism of voriconazole may also be involved in increasing photosensitivity in affected individuals [179].
Voriconazole phototoxicity is not a limited benign cutaneous reaction as several cases of squamous cell carcinoma as well as in situ melanoma arising within chronic phototoxic lesions, have been reported secondary to voriconazole photocarcinogenesis [180–183]. Patients who are prescribed voriconazole should be made aware of these risks and must be educated about the importance of aggressive photoprotection. In patients who develop phototoxicity, voriconazole should be substituted with an alternative antifungal, such as posaconazole. Physicians caring for SOTR should consider alternatives to voriconazole altogether in patients at high risk for skin cancer [184].
1.9.3 EGFRI
Skin cancer, in particular squamous cell carcinoma (SCC), is a well-known consequence of long-term immunosuppression [40, 185–187]. Epidermal growth factor receptor inhibitors (EGFRI) can block the proliferation of undifferentiated basal epidermal keratinocytes and, therefore, can be used as a targeted chemotherapy in head and neck squamous cell carcinomas [184, 188, 189]. EGFRI work either by targeting the extracellular EGFR domains (cetuximab and panitumumab) or by inhibiting the intracellular EGFR tyrosine kinase (erlotinib, gefitinib, and lapatinib) [190]. Such targeted therapies may provide a promising strategy for the treatment of patients with aggressive cutaneous SCC and are being investigated in clinical trials. Most common adverse effects with EGFR blockade are skin toxicities. Within the first 2–4 weeks of therapy, over 50 % of patients taking EGFRI develop a dose-dependent acneiform eruption, consisting of non-comedonal, erythematous papules and pustules that primarily involve the head, neck and upper trunk, and extremities [191, 192]. The eruption typically improves after reaching greatest activity within 3 weeks and, thus, discontinuation of the therapy is not usually required. In fact, the incidence and severity of the acneiform eruptions correlate with a better response to EGFRI therapy [193]. Xerosis is a common finding in patients on EGFRI therapy; therefore, moisturizing and mild cleansing of the skin are important. Other treatments include topical erythromycin, metronidazole, fusidic acid, zinc shake lotion, and doxycycline [193, 194]. Topical retinoids are not recommended because of their propensity to exacerbate dryness and irritation [195].
1.10 Summary
Management of cutaneous disorders in STOR involves both medical and surgical disciplines within dermatology. This population is at greater risk of cutaneous infections, inflammatory disorders, and skin cancer. Specialty dermatology clinics can be instrumental in addressing the increased incidence of skin disorders, both with diagnosis and treatment, when effective therapies exist. Informative counseling can also be of utmost importance in managing disease expectations and burden. Finally, when appropriate the dermatologist may have to recommend decreasing immunosuppressive therapy and should be able to determine the appropriate timing for this potential change. Functioning as part of the multidisciplinary team is of fundamental importance to successful management and outcomes for SOTR.
References
1.
Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2011 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [11/5/2013]. Available from: http://srtr.transplant.hrsa.gov/annual_reports/2011/.
2.
Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–58. PubMed PMID: 1590990. Epub 1992/01/01. eng.PubMed
3.
Beiras-Fernandez A, Thein E, Hammer C. Induction of immunosuppression with polyclonal antithymocyte globulins: an overview. Exp Clin Transplant. 2003;1(2):79–84. PubMed PMID: 15859913. Epub 2005/04/30. eng.PubMed
4.
Mottershead M, Neuberger J. Daclizumab. Expert Opin Biol Ther. 2007;7(10):1583–96. PubMed PMID: 17916050. Epub 2007/10/06. eng.PubMed