7 Recent trends in kidney transplantation
a review
Introduction: the wages of success
As kidney transplantation enters its sixth decade, the challenges facing the field are largely the consequence of its success as a therapy. It is difficult to remember (and for those under a certain age, an experience not available for recollection) the era when the physiological burden of systemic immune suppression was so taxing as to make kidney transplantation a dubious alternative to dialysis. For some time, this has not been the case. At least since the advent of ciclosporin A in 1983, the outcomes for kidney transplantation have been shown to be vastly superior to dialysis for virtually every category of patient with end-stage renal disease (ESRD).1
 Indeed, while survival among dialysis-dependent patients has not changed appreciably in almost two decades (with only 35% alive after 5 years), survival of kidney transplant recipients is now in excess of 90% at 3 years, with a documented advantage over dialysis even among elderly recipients.2,3
Indeed, while survival among dialysis-dependent patients has not changed appreciably in almost two decades (with only 35% alive after 5 years), survival of kidney transplant recipients is now in excess of 90% at 3 years, with a documented advantage over dialysis even among elderly recipients.2,3
Current challenges in kidney transplantation, then, might best be characterised as a consequence of technological progress.4 While clinical hurdles remain in the attempt to deliver the very best outcomes for recipients, the most pressing issues are now driven by the limiting factor of organ availability. The question is no longer who might benefit from transplantation (answer: nearly everyone), but who shall benefit, when not everyone can. Indeed, it is this seemingly straightforward, pedestrian problem that underlies much of the recent public policy debate in renal transplantation, and that fuels unsavoury practices such as transplant tourism.5 General approaches to the issue can be loosely classified into:
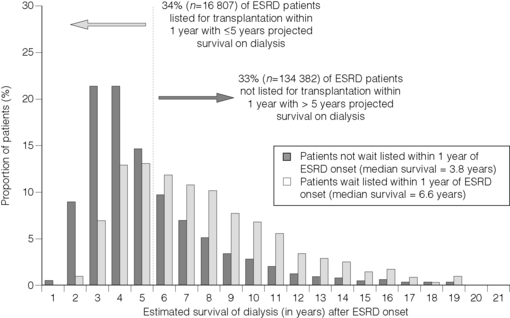
 In contrast, a recent analysis by Schold et al. found that, by applying straightforward demographic criteria defining transplantability, 16 807 Americans might indeed be inappropriately listed. The quandary, however, is that these same criteria identified over 134 000 others on dialysis who would benefit from transplantation, but are not currently on the waiting list (Fig. 7.1).9
In contrast, a recent analysis by Schold et al. found that, by applying straightforward demographic criteria defining transplantability, 16 807 Americans might indeed be inappropriately listed. The quandary, however, is that these same criteria identified over 134 000 others on dialysis who would benefit from transplantation, but are not currently on the waiting list (Fig. 7.1).9
 Using retrospective data collected on deceased donors and recipients, this new system would allocate organs based on a scoring system designed to maximise ‘life years following transplant’ or ‘LYFT’. In so doing, the proponents suggest, scarce organs would be put to better use, and the opportunity to maximise LYFT would offer a clearer metric (in the way time spent on a waiting list does not) to adjudicate competing claims for the same organ.10
Using retrospective data collected on deceased donors and recipients, this new system would allocate organs based on a scoring system designed to maximise ‘life years following transplant’ or ‘LYFT’. In so doing, the proponents suggest, scarce organs would be put to better use, and the opportunity to maximise LYFT would offer a clearer metric (in the way time spent on a waiting list does not) to adjudicate competing claims for the same organ.10
Though the access issue may seem paramount, and advances in immunosuppression and overall care have transformed the act of transplanting an organ from audacious to routine, rejection is still a problem, and further improvement is most assuredly desirable. Even so, state-of-the-art therapies have made it increasingly difficult to test new approaches that, in the short term at least, may demand deviation from accepted orthodoxy. At the same time, rather than the traditional problem of cellular rejection, it now appears that antibodies and B cells are increasingly the source of clinical conundrums, and the highly sensitised recipient continues to pose daunting clinical challenges.11 These trends have revitalised the science of histocompatibility, rendered briefly moribund by the use of potent induction and maintenance immunosuppression. In this chapter, we will summarise these emerging trends, focusing on efforts to improve both access to and outcomes from kidney transplantation.
Kidney transplantation: the treatment of choice for end-stage renal disease
As recently as two decades ago, the best treatment option for patients with end-stage renal disease (ESRD) was a debatable question. Innovations such as recombinant erythropoietin and biocompatible membranes dramatically reduced the comorbidity associated with chronic haemodialysis. Peritoneal dialysis offered relative freedom from dietary restrictions and thrice-weekly visits to dialysis units. Although the overall mortality rates for those on dialysis appeared stable, given a substantially older patient population, outcomes were clearly improving.2,12,13 Transplantation, despite its life-enhancing qualities, was haunted by the complications associated with toxic or inadequate immunosuppression, including rejection, osteonecrosis, opportunistic infections and cancer.14 Almost half of deceased donor kidneys were lost within 3 years after engraftment. Rennie’s 1978 contention that a kidney transplant was merely a ‘temporary respite’ from dialysis remained an accurate, if depressing, assessment.15
In the last decade, advances in transplantation have increasingly shifted the debate over optimal ESRD treatment modality from an open question to an established conclusion. After a decade of stagnant graft survival rates, the statistical half-life of allografts began improving in 1988, and by 1996 had almost doubled.16 While still associated with substantial morbidity, new options in maintenance immunosuppression lessened rejection rates and reliance on corticosteroids, reducing complications after transplantation.17 In the USA, where the Social Security Amendments of 1972 had made payment for ESRD therapy an entitlement for most of the population, transplantation proved substantially less expensive than maintenance dialysis.18 Finally, a new statistical approach made it possible to better compare outcomes with dialysis and transplantation, a comparison previously difficult because of a bias in modality selection.19 These recent analyses have defined a survival benefit relative to maintenance dialysis for virtually all ages and categories of patients with ESRD. Confirming the widely held notion that ESRD is a systemic disease conferring significant physiological deterioration over time, longevity on dialysis is now considered a major risk factor for graft loss: those patients transplanted earliest in the course of ESRD experience the best outcomes.20 Just how early a recipient might be transplanted and still derive survival benefit, compared to accruing the cardiovascular vicissitudes of chronic kidney disease, remains an open question.
Why such significant changes in favour of renal transplantation? Better allograft survival is the result of several advances. More sensitive crossmatch techniques, in particular single-antigen flow-bead assays, have made donor–recipient compatibility easier to determine, even while perhaps reducing the rates at which sensitised recipients are transplanted.21 Twenty-five years ago, the immunosuppressive armamentarium included only polyclonal antilymphocyte globulins (useful for only the short term), azathioprine (relatively ineffective) and large doses of corticosteroids. An improved understanding of the immunobiology of rejection has produced agents with greater specificity and less toxicity,22 though intriguing recent data from the MYSS trial raise the question of the relative benefit of the newer agents.23 The latter point is of considerable interest, given the cost differences between mycophenolate mofetil and azathioprine.
Under current immunosuppressive protocols, fewer than 25% of patients now experience acute rejection, and immunological graft losses are uncommon early after transplantation.17,22 Recent data indicate that quality and stability of renal function (as reflected in the serum creatinine) in the early post-transplant period is a strong predictor of long-term graft survival.24 Current immunosuppression is associated with unprecedented preservation of glomerular filtration rate.25 Immunosuppressive complications have become both less common and more manageable. Improved antiviral, antibacterial and antifungal agents have reduced morbidity and mortality due to infections. The adoption of prophylactic and pre-emptive approaches to antimicrobial therapies has even reduced the incidence of serious infections among transplanted patients.
Dialysis patients, on average, have 20–25% the life expectancy of healthy, age-matched controls.2 The most common causes of death are cardiovascular and infectious. While transplant recipients remain at greater risk of cardiovascular and infectious deaths than the general population, transplantation confers a substantial risk reduction relative to remaining on dialysis, and manoeuvres to reduce cardiovascular risk, including treatment of hyperlipidaemia, are proving to be efficacious after transplantation.26,27 Recent reports indicate risk of death from common malignancies (lung, breast, colon, etc.) is nearly identical among those on dialysis and those with functioning transplants.28 While the risk of dying in the perioperative period exceeds that of a waiting-list patient on dialysis, mortality risks decline over time, equalising after about 3 months. After that time, the transplanted patient is at substantially less risk of death. Life expectancy with a deceased-donor transplant ultimately is about twice that of remaining on dialysis.19 In the USA, 70% of patients with ESRD are currently undergoing dialysis. However, of those patients living at least 10 years with ESRD, 70% have functioning allografts.2 Kidney transplantation saves lives, a newly delineated fact that is increasingly appreciated by patients with ESRD.
Supply and demand
The number of identified dialysis-dependent ESRD patients in the USA has grown substantially over the last two decades. In 2005, 341 000 patients in the USA suffered from ESRD. Current estimates vary, but that number is expected to grow to between 400 000 and 520 000 by 2010, and approach 525 000–700 000 by 2020.2,29,30 Today, in the USA, there are over 73 000 people waiting for a kidney transplant from a deceased donor, and by 2010 the waiting list is expected to grow to nearly 100 000 candidates. In 2006, some 6570 recipients, or 8% of the total listed, died waiting and 2020 patients were removed after being deemed ‘too sick to transplant’.31 Furthermore, the extended time waiting on dialysis exacts a toll. While receiving a renal transplant, even one of marginal quality, confers a significant mortality benefit compared to remaining on dialysis, the progression of cardiovascular morbidity while on dialysis translates into inferior allograft and patient outcomes.19,20,32 Utilising a multivariate analysis, Meier-Kriesche et al. demonstrated that any amount of time on dialysis results in inferior graft outcomes when compared to pre-emptive transplantation.20
 Annual mortality on the waiting list is 10.8% for diabetics and 6.3% for non-diabetics.33 While this is better than for other non-transplanted patients with ESRD, it also means that a substantial number of patients will die awaiting transplantation.
Annual mortality on the waiting list is 10.8% for diabetics and 6.3% for non-diabetics.33 While this is better than for other non-transplanted patients with ESRD, it also means that a substantial number of patients will die awaiting transplantation.
Currently, a ‘low-risk’ patient without diabetes who is less than 65 years of age at the time of listing has a greater than 30% chance of dying before being offered a transplant.34
Even as some observers report a levelling off in the number of incident patients with ESRD, recent reports suggest that the rate of growth of chronic kidney disease (CKD) continues unabated.35 Since the vast majority of patients with CKD die from accumulated cardiovascular complications prior to reaching ESRD, at least one explanation for the current and future growth in the number of patients with ESRD is success in treating hypertension, diabetes and other risk factors that may slow progression of heart disease. The attendant irony of this success is that a small but growing fraction of patients are now living long enough to progress to stage V CKD and thus require dialysis or transplantation.36
The financial costs of these trends command attention. In the USA, federal expenditures on ESRD alone amount to US $21 billion in 2005, accounting for 6.4% of the Medicare budget for that year, spent on 0.6% of eligible Medicare beneficiaries.2 Of that $21 billion, only $0.59 billion (2.8%) was spent on organ acquisition and transplant surgical costs. Recent estimates have suggested that the break-even point between the cost of dialysis and transplantation may now be less than 1.5 years ($101 259 for 1.5 years of dialysis versus $85 507 after 2 years of transplantation and full reimbursement for immunosuppressant medications.37 Although current US statutes mandate transplant evaluation for all patients with ESRD, the previously cited study of Schold et al. indicates that there are as many dialysis patients not listed as listed.9
Increasing the supply of organs
Expanded criteria organs
In the USA, numerous efforts have been made in the last several years to maximise organ procurement from deceased donors. These efforts have occurred under the aegis of the Organ Donor Collaborative, which has sought to identify best practices by the most successful organ recovery agencies and promulgate them to others. As a consequence of these efforts, the number of deceased-donor organs has increased by about 10%. The largest growth, by category, has been in kidneys from extended criteria donors. As has often been the case in organ recovery from deceased donors, Spain appears to have been leading the way.38 A conference convened to address the shortage of donated organs found that the principal difference between Spain and the USA was utilisation of donors older than 45 years of age.39 Subsequently, a new category of donors was defined as ‘expanded criteria’ donors (ECDs). Previously, a high percentage of these organs were either discarded (18%) or never recovered. Donor factors utilised to define ECD kidneys were known to be independently associated with an increased risk of graft loss, and included donor age greater than 60 years, cerebrovascular accident as cause of death, hypertension and baseline renal insufficiency (serum creatinine greater than 1.5 mg/dL).40,41 Despite a graft failure rate 70% greater than kidneys from standard donors, Ojo and colleagues demonstrated a substantial survival benefit from these kidneys relative to remaining on dialysis.33
 Recent analysis of the ECD programme (initiated in the USA in 2002) documented increased access to transplantation for those candidates willing to accept ECD kidneys, with acceptable outcomes.32
Recent analysis of the ECD programme (initiated in the USA in 2002) documented increased access to transplantation for those candidates willing to accept ECD kidneys, with acceptable outcomes.32
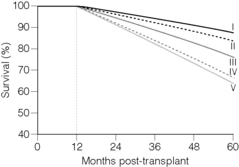
Retrospective study of outcomes from organs turned down at one institution and used at another have helped to refine what is known about donor quality, and thereby salvage organs that might otherwise never have been used. Still, a recent examination of the data suggests that the category of ‘extended criteria’ encompasses a wide range of donor quality, so the hopeful outcomes derived from some ECD kidneys suggest that at least some organs so classified tend to offset considerably less sanguine outcomes from the majority of ‘expanded criteria’ donors42 (Fig. 7.2). As we predicted in the previous version of this chapter,40 the increased use of ECD organs has accounted for most of the 10% increase in the number of organs available in recent years. Which cohorts of recipients would most benefit from these kidneys remains a topic of intense interest, and will attract more attention if the allocation system undergoes revision that emphasises matching donor–recipient pairs to maximise life years gained following transplantation.10,43–45
Donation after cardiac death
Still another, though substantially smaller, source of organs has come with the rehabilitation of donation after cardiac death (or DCD), previously known as non-heart-beating donation. DCD can be either ‘controlled’, in which the donor’s cardiopulmonary arrest spontaneously occurs in an operating room after cessation of life-sustaining measures in a critically ill patient, or ‘uncontrolled’, in which a patient sustaining an out-of-hospital cardiopulmonary arrest, and in which cardiopulmonary resuscitation is not successful, is placed on cardiopulmonary bypass until consent is obtained (or not) for organ procurement. Use of DCD in the USA, and most of the Western world, is essentially all in the controlled situation, and outcomes from these kidney donors appear comparable to those from standard criteria donors.46
Uncontrolled donation after cardiac death currently occurs with any regularity only in Spain.47 In its recent effort to address the growing disparity between the demand for and supply of organs for transplantation, the Institute of Medicine settled on uncontrolled donation after cardiac death as a signature solution, with the promise of yielding as many as 22 000 kidneys per year.48 Left out of the discussion were the numerous significant logistic and ethical difficulties associated with a widespread programme of recovering organs from uncontrolled DCD situations. Such a programme would necessitate:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree