Fig. 11.1
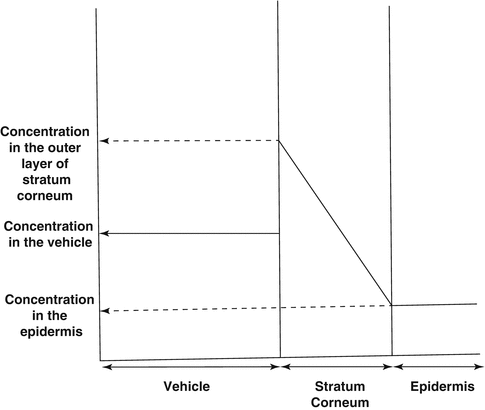
Diagram showing the critical degree of supersaturation (DS) and the different stability states of supersaturated systems
The advantages of supersaturated systems for percutaneous penetration were first recognized by Higuchi (Higuchi 1960) (Fig. 11.2). The role of supersaturation in transdermal delivery has been tested and found effective consistently in the past (Coldman et al. 1969; Guy 2007; Morrow 2007); however, in order to achieve supersaturation, the choice of optimal solvents or co-solvents is critical (Poulsen et al. 1968). The concept and the understanding of supersaturated systems were borne out of the crystallization theory, where a solution must be first of all supersaturated in order for crystals to form. The precise mechanism of nucleation and subsequent crystal growth is not fully understood. There are multiple theories proposed in the literature for predicting crystal growth (Frank 1949; Mehta et al. 1970; Rodríguez-Hornedo and Wu 1991). It has been observed that the choice of solvent is critical for the type of crystals (e.g., hydrate, alcoholate) and polymorphs formed in the solution (Corrigan and Timoney 1974; Khoshkoo 1991). It has also been observed that different polymorphs of a compound can have different saturated solubilities in a solvent and, therefore, one can be supersaturated with respect to the other. Solvate forms of drugs, sometimes called pseudopolymorphs, can also have different solubilities and dissolution rates to their corresponding non-solvate forms. Similar to polymorphs, an anhydrous form of a drug can be supersaturated with respect to its hydrate form (de Smidt et al. 1986; Davis 1993).
11.2 Method of Preparation for Supersaturated Solutions
There are four primary methods of preparing the supersaturated solutions, viz., biphasic or binary mixing (addition of a substance to a solution which reduces the solubility of the solute); evaporation; heating and then cooling; and via chemical reactions (of two or more solutes to produce a new compound which is less soluble in solution than the original starting solutes), e.g., melt extrusion technology, granulation. The supersaturated solutions prepared using all the above techniques have shown increased percutaneous permeation of drugs in the past (Adrian et al. 2002).
The preparation of biphasic mixtures can be explained by constructing a curve for the saturated solubility of the drug in a binary co-solvent system where the drug is more soluble in one of the solvents than the other (Fig. 11.3). By preparing a saturated solution of the drug in solvent B and diluting with solvent A, a point, E, is obtained along the line CD, where C represents 100 % of solvent A and D represents 100 % solvent B. This is a supersaturated solution and its degree of saturation is calculated by dividing the amount of drug in solution by its saturated solubility in the same co-solvent mixture. In this example, the solution E has two degrees of saturation. Crystallization in a biphasic system is inhibited (or retarded) by addition of an anti-nucleant polymer to solvent A (Adrian 2002).
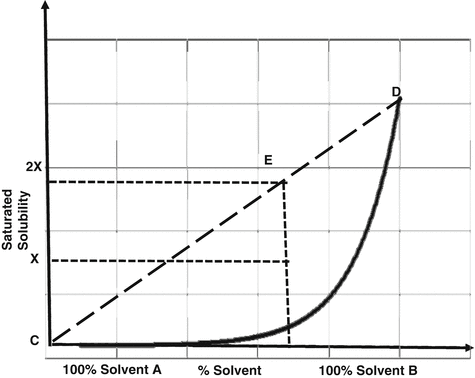

Fig. 11.3
Graph showing the solubility of a drug in a binary co-solvent system and the method used to obtain a supersaturated solution
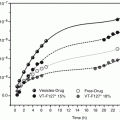
The diffusion studies of the biphasic mixture of water to the solutions of hydrocortisone acetate in propylene glycol (PG) and the anti-nucleant polymers polyacrylate, PVP, and HPMC indicated that the supersaturated solutions gave an increase in flux when compared with the saturated solutions and that the flux was proportional to the degree of saturation. It was also found that a 0.02 % supersaturated gel of hydrocortisone acetate was bioequivalent to a 1 % cream in reducing a surfactant-induced erythema (Davis and Hadgraft 1991; Davis 1993). Similar observation with increase in flux from increasing DS has also been observed in fentanyl delivery when supersaturated solutions with different DS were prepared using PG/water and PG/ethanol systems (Santos et al. 2011). An 18-fold increase in the concentration of estradiol in the stratum corneum was observed within ten minutes of exposure to a supersaturated solution when compared with a saturated solution in the same co-solvent mixture (Megrab et al. 1995). A testosterone-supersaturated solution with 2.5° of supersaturation in 1/1 ethanol/PG was found to show flux (across hairless rat skin) similar to that of metered dose transdermal spray system under commercial development (MDTS®, Acrux Ltd., Australia, www.acrux.com.au) (Leichtnam 2006). The delivery of piroxicam from a fourfold supersaturated solution across silicone and human skin in vitro was increased four times compared to the drug delivery of a saturated solution in the same vehicle. The solutions up to 4° of saturation were found to be stable for 16 h and then crystallization was observed (Pellett et al. 1997). It was also demonstrated via tape stripping technique that the intercellular regions of the stratum corneum were capable of maintaining supersaturated systems as the drug is transported across the stratum corneum (Pellett et al. 1997). In another study with delivery of fluocinonide across silicone membranes using in vitro diffusion cells, supersaturated solutions up to 3.8° of saturation were prepared using PVP and a vehicle composition of ethanol, water, glycerol, and propylene glycol, and it was found that fluocinonide permeation across silicone membranes was linearly related to degree of saturation (Schwarb et al. 1999). Permeation studies of ketotifen from silicon-based pressure-sensitive adhesive (PSA) with n-hexane and another solvent dichloromethane, tetrahydrofuran, acetone, ethyl acetate, or toluene demonstrated that the formulation was in a supersaturated state (Inoue et al. 2005).
Supersaturation can also be achieved by evaporation of the volatile components in the formulation resulting in decreased solubility in the residual component of the skin surface leading to increased thermodynamic activity. However, the only drawback of supersaturation achieved via evaporation is the lack of control on concentration of the supersaturated solution thereby having less control on flux via that solution. The evaporation theory can be tested by occluding/un-occluding the formulation when volatile solvents are present in the vehicle (Tanaka et al. 1985). Just like binary systems, control of crystallization is important to maintain the supersaturated state of drug in solution. In a study where nifedipine was dissolved in combination of vehicles like isopropyl myristate, PG, and acetone and studies for permeation across an ethylene-vinyl acetate copolymer membrane, the initial flux values in the system were found to be greater than the final steady-state fluxes attributing to the crystallization of the drug component. The incorporation of anti-nucleant polymers into the IPM:PG:acetone vehicle led to a three to five times enhancement in flux (Kondo and Sugimoto 1987). These findings of increased permeation across the lipoidal membrane were confirmed in an in vivo/in vitro correlation study where similar observations were reported across excised male Wistar rat skin in vivo using the nifedipine solution in 75:25 ethanol and diethyl sebacate combinations (Kondo et al. 1987). In another study, hydrocortisone was dissolved in a mixture of acetone and water, and up to 3.9° of saturation was achieved giving a proportional increase in flux, which was monitored across an ethylene-vinyl acetate copolymer membrane after the evaporation of volatile component (Theeuwes et al. 1976). The increase in delivery of sodium nonivamide acetate has also been tested across rat skin from supersaturated solutions prepared by evaporation of the volatile components of an aqueous ethanolic vehicle, and two components, methyl cellulose and hydroxypropyl cellulose, were found to have a stabilizing effect as an anti-nucleant preventing crystallization within the skin membrane (Fang et al. 1999).
When a solution is prepared from cooling it to a temperature below that of the saturated solubility, the solution may crystallize or a stable supersaturated system may result. A tenfold increase in the permeation was observed when an indomethacin gel was prepared by heating a mixture of the drug with hydrogenated soybean phospholipid and liquid paraffin to 95 °C, cooling to room temperature, placing it in an air incubator at 40 °C for 3 days, and then storing at room temperature, compared to that prepared at room temperature in the same vehicle components. Supersaturated solutions of flurbiprofen and ketoprofen also showed an increase in the permeation rates from the heated and cooled formulations, but no difference in the rates for ibuprofen formulations was observed (Henmi et al. 1994).
11.3 Role of Anti-nucleant Polymers
Supersaturated systems, by definitions, are unstable in their native state. In order to stabilize a supersaturated system and to achieve effective transdermal enhancement, it is absolutely crucial to keep the process of crystal formation to its minimum. For this purpose, anti-nucleant polymers are used in the supersaturated system. Some of the common polymers used as effective anti-nucleants are hydroxypropyl methyl cellulose (HPMC), polyvinyl pyrrolidone (PVP), polyvinyl alcohol (PVA), polyethylene glycol (PEG), and Eudragits (acrylic polymers). These polymers have been shown to inhibit the crystal growth of compounds like paracetamol, nifedipine, spironolactone, sulfamethiazole, hydroflumethiazide, n-paraffin, estradiol, and griseofulvin from supersaturated solutions in a particular solvent and delivery systems (Simonelli et al. 1970; Corrigan and Timoney 1975; Sekikawa et al. 1978; Holden 1979; Hasegawa et al. 1985; Femi-Oyewo and Spring 1994; Kotiyan and Vavia 2001; Valenta and Auner 2004). Use of copolymers of methacrylic acid (Eudragit® E, EuE, and Eudragit® RL, EuRL) as ibuprofen crystal inhibitors in the matrices was found to prevent the drug crystallization for more than 12 months (Cilurzo et al. 2005). In another study, supersaturated solutions of ibuprofen were prepared using 25 % PG, 5 % vitamin ETPGS (d-alpha tocopheryl polyethylene glycol 1,000 succinate), and 5 % ethylene oxide/propylene oxide block copolymer (pluronic F127) solvents and their combinations to achieve different DS (0.5, 1, 2.5, 5.0, 10.0, 25.0, and 50), and it was found in that study that vitamin ETPGS improved the flux better compared to PG and pluronic F127. The optimization of the vitamin ETPGS/ibuprofen formulation with polymeric stabilizers like HPMC and PVP K-30 resulted in inhibiting crystal growth (HPMC showed better crystal growth inhibition compared to that of PVP K-30). However, the use of PVP K-30 increased the permeation rate of drug through the skin relative to the HPMC (Ghosh and Michniak-Kohn 2012).
How to select an appropriate polymer for a particular drug-solvent system is of a concern. The concept of nucleation and crystallization is not completely understood but efforts have been made to study the process using techniques like differential scanning calorimetry (DSC). In a study involving isothermal crystallization of lidocaine (LC) in supersaturated polyacrylate pressure-sensitive adhesive (LC/Duro-Tak® 87-2287 (DT2287)) system, some of the common reasons for crystallization are given (Cui and Frank 2005). Multiple theories have been proposed for the inhibition of crystal formation by the use of polymer additives and therefore stabilization of supersaturated solution of a compound in a solvent. In a study involving the preparation of supersaturated sulfamethiazole solution in presence of PVP, it was suggested that PVP formed a netlike structure over a growing crystal face with fingerlike crystal growths occurring between the pores of the PVP network, and due to the curvature of these protrusions, a higher degree of supersaturation was required to continue crystal growth (Simonelli et al. 1970). In another study involving the investigation of crystallization and morphology of hydrocortisone acetate crystal formation, it was proposed and observed via IR spectroscopy that crystal growth was inhibited by absorption of polymer, PVP into the growing crystal surface through hydrogen bonding (Raghavan et al. 2001). Similar observation of presence of hydrogen bond was also observed in an ibuprofen – HPMC system (Iervolino et al. 2001). In yet another study, the preparation and stabilization of colloidal dispersions of triclosan, an antimicrobial agent used up to 2 % in cosmetic and detergent formulations for disinfection of the skin, were performed from supersaturated solutions of triclosan either in the absence or presence of the polymer, HPMC, and it was observed that the particle sizes of triclosan were stabilized in the range of 90–250 nm after addition of HPMC in a water-propylene glycol co-solvent system, whereas large-shaped crystal morphology was observed in the absence of polymer (Raghavan et al. 2003). Stabilization of fentanyl in the supersaturated PG/water formulation with 3DS was achieved by addition of 1 % hydroxypropyl cellulose (HPC) leading to improved permeation flux across the skin. Fentanyl crystal growth was retarded minimally using HPMC or PVP K90 at 1 % (w/v) for the 5 DS formulation compared with HPC (Santos et al. 2011).
However, presence of a polymer in the drug solution does not necessarily affect drug permeation in all cases. In a study of the effect of hydroxypropyl-β-cyclodextrin (HPβCD) on in vitro transdermal permeation of corticosterone through hairless mouse skin, contradictory evidences have been observed between the groups. Shaker et al. have been observed that use of HPβCD, PVP, or the HPβCD/PVP combination does not affect the permeation of corticosterone across synthetic cellulose membrane or the hairless mouse skin, whereas Loftson et al. have reported an increase in permeation of corticosterone in presence of HPβCD (Loftsson and Sigurðardóttir 1994; Shaker et al. 2003; Torres-Labandeira et al. 1991). Similarly, use of 2-hydroxypropyl-ß-cyclodextrin has also been found not to alter crystal growth in ibuprofen solution (Iervolino et al. 2001). A very similar observation was observed in a study of testosterone, added in excess to 4:1:1 (v/v) ethanol/PG/water along with multiple anti-nucleant polymers (PVP Kollidon®30, vinylpyrrolidone-vinyl acetate copolymer Kollidon®VA64, a randomly methylated cyclodextrin RAMEB, methacrylic acid and ethyl acrylate copolymer Eudragit®L100-55, nonionic poly (ethylene oxide) polymer Polyox®WSR N-10, hydroxypropyl methyl cellulose Methocel®E5, hydroxypropyl methyl cellulose phthalate HPMCP®50, and hydroxypropyl cellulose Klucel®EF) to prevent crystallization. It was found that only Kollidon®VA64 and a cyclodextrin derivative, RAMEB, at 5 % conc., showed promise in the preparation of supersaturated solution with a degree of supersaturation between 1.4 and 2.6, but the supersaturated state existed only for 6 h (Leichtnam et al. 2006).
11.4 Determination of Supersaturation and Crystal Growth
The easiest way to determine crystal formation is via spreading the solution into a thin film on a slide and visually observing the crystals under the microscope. Other methods to determine the solubility of the drug and crystal formation are via methods like isothermal heat conduction, microcalorimetry, X-ray diffraction, and DSC (Theeuwes et al. 1974; Latsch et al. 2004a, b). Along from experimental techniques, computational techniques like kinetic Monte Carlo method has been used for predicting of Oswald ripening and evolution in crystal formation/growth of a supersaturated solution (Zeng et al. 2004, 2006).
11.5 Synergistic Use of Supersaturation Along with Other Enhancement Technologies/Methods
Synergistic effect of supersaturation along with the use of the chemical enhancer, oleic acid, was observed on the flurbiprofen permeation across human skin. The stratum corneum of human skin was pretreated for 1 h with a 2.8 % ethanolic (EtOH) solution of oleic acid (OA), and a supersaturated solution of flurbiprofen with six degrees of saturation was applied on the skin. Compared to ER=1.0 with saturated solution of flurbiprofen in EtOH, the 2.8 % OA in EtOH showed an ER of 2.1, whereas the sixfold supersaturated solution showed an ER of 4.5, whereas in presence of both supersaturation and oleic acid, the enhancement ratio (ER) of 9.9 was observed (Pellett 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree