Chapter 55 Therapeutic photomedicine
1. What is phototherapy?
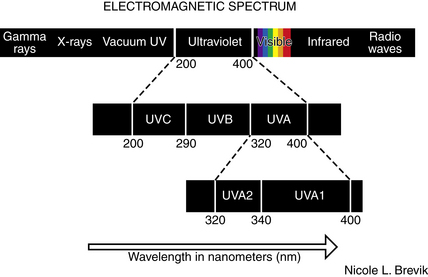
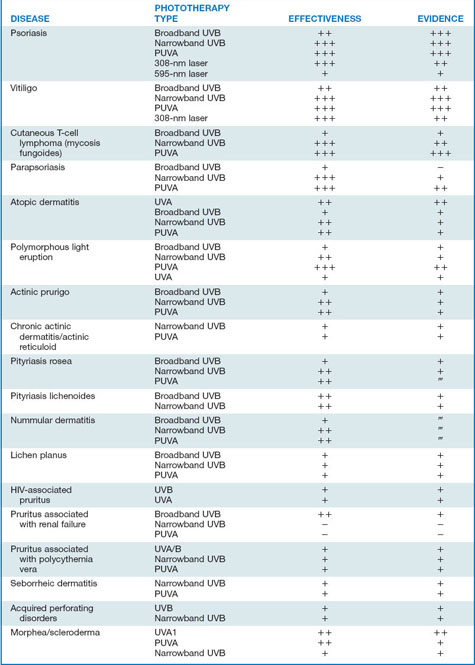
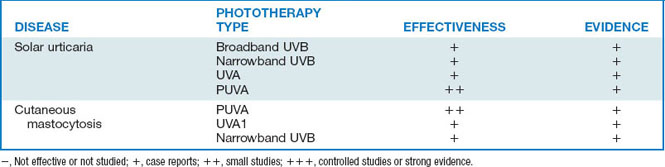
Phototherapy is the use of nonionizing electromagnetic radiation (usually in the ultraviolet [UV] range, but also extending into the visible light range) to treat cutaneous disease (Fig. 55-1).
3. How does traditional phototherapy work?
Immunomodulation locally, and possibly systemically, is the primary mechanism of action of phototherapy. Effects on keratinocyte proliferation and differentiation are likely secondary to the immunomodulatory effects. Immunomodulatory effects include: T-cell apoptosis (CD8+ in epidermis, CD4+ in dermis, CD25+ in both epidermis and dermis), inhibition and depletion of antigen-presenting cells (Langerhans cells in epidermis, dermal dendritic cells in dermis), release of immunosuppressive cytokines (interleukin [IL]-10 and IL-4, reduced expression of tumor necrosis factor–α [TNF-α]), interferon (IFN)-γ, and IL-12 locally, photoisomerization of trans-urocanic acid to cis-uracanic acid (which suppresses cellular immune responses, such as antigen presentation by Langerhans cells), and upregulation of CD95L (Fas ligand that binds to the Fas receptor on T cells inducing apoptosis).
Walters IB, Ozawa M, Cardinale I, et al: Narrowband (312-nm) UV-B suppresses interferon gamma and interleukin (IL) 12 and increases IL-4 transcripts: differential regulation of cytokines at the single-cell level: Arch Dermatol 139:155–161, 2003.
4. How is phototherapy administered?
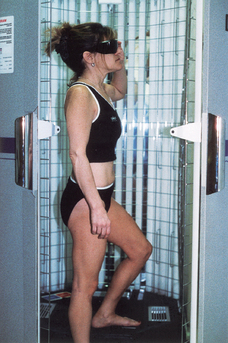
It is usually administered in a physician’s office or treatment center. Less commonly, patients purchase UVB phototherapy units and treat themselves at home. Patients receive a controlled dose of ultraviolet light while standing in a booth lined with high-output ultraviolet light bulbs (Fig. 55-2). The initial ultraviolet light exposure is determined by assessing the patient’s skin type and history of burning or by establishing the minimal erythema dose (MED).
5. Compare the induction phase, maintenance phase, and tapering phase for various forms of phototherapy.
• BBUVB: Daily treatments, increasing approximately 10% each treatment until pink or therapeutic level reached
• NBUVB: 3 times weekly, increasing approximately 10% each treatment until pink or therapeutic level reached
• UVA1: Daily or 3 times weekly, starting at 5 to 10 J/cm2, usually set at a standard dose depending on condition being treated. In general, high-dose regimens (130 J/cm2), medium-dose regimens (30 to 50 J/cm2) and low-dose regimens (20 J/cm2) have been used.
6. What advantages does narrowband UVB have over broadband UVB?
Narrowband UVB utilizes a TL-01 light source emitting UV light almost exclusively at 311 nm, which is near the most effective action spectrum for induction of T-cell apoptosis in inflammatory skin diseases such as psoriasis. The absence of other wavelengths of UV light decreases side effects (erythema, carcinogenesis, photoaging) without sacrificing efficacy. Unlike broadband UVB, suberythemogenic doses are effective.
7. What advantages does narrowband UVB have over PUVA?
Narrowband UVB does not have the risks, side effects, and inconvenience of taking oral psoralens, including nausea, vomiting, dizziness, headache, insomnia, depression and anxiety, hepatotoxicity, drug interactions, allergic reactions, photoallergic reactions, PUVA keratoses, PUVA lentigines, PUVA pain, transient nail pigmentation, photoonycholysis, facial hypertrichosis, licheoid eruptions, photosensitivity for 24 hours (even through windows), risk of cataracts, increased risk of melanoma and other skin cancers including genital skin cancers, narrow window of treatment after taking medication (1 to 2 hours), variability of tissue response depending on medication absorption and distribution (which is time sensitive), and need to wear sunglasses and avoid natural sunlight after taking medication for 24 hours. Narrowband UVB has not demonstrated increased carcinogenicity, thus far in humans, and the efficacy is similar. However, increased carcinogenicity has been noted in rats, and long-term follow-up is not yet available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree